The synthetic method of favipiravir
A favipiravir and synthesis method technology, applied in the field of drug synthesis, can solve problems such as hydrogen peroxide aggregation and explosion, and achieve the effects of reducing pollution and loss, high production efficiency, and high mass transfer efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
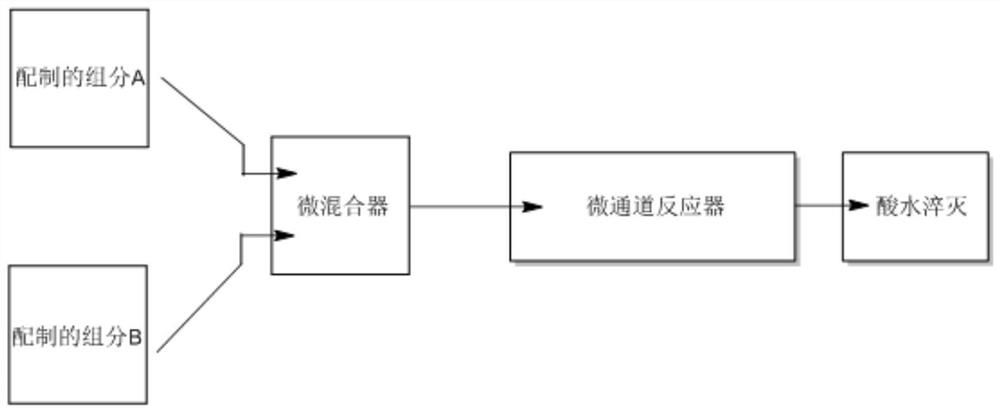
[0035] A method for synthesizing favipiravir using a micromixer and a microchannel reactor in combination, the reaction flow is as follows figure 1 As shown, the reaction formula is as follows:
[0036]
[0037] Include the following steps:
[0038] 1. Prepare the solution:
[0039] Prepare 6-fluoro-3-hydroxypyrazine-2-carbonitrile (10g, 72mmol), sodium hydroxide (2.85g, 72mmol), 72ml of water (1mol / l), and mix the three reagents to prepare component A ;
[0040] Prepare hydrogen peroxide (16.3 g, 144 mmol), sodium hydroxide (2.85 g, 72 mmol), and 72 ml of water (1 mol / l), and prepare component B by mixing the three reagents.
[0041] 2. Set the injection pump parameters:
[0042] Set injection pump 1 (injection pump 1 is connected to solution A) injection rate, 25 degrees Celsius, 0-1Mpa,
[0043] Set the injection rate of the injection pump 2 (the injection pump 2 is connected to the solution B), 25 degrees Celsius, 0-1Mpa.
[0044] 3. Screening the Micromixer:
[...
Embodiment 2
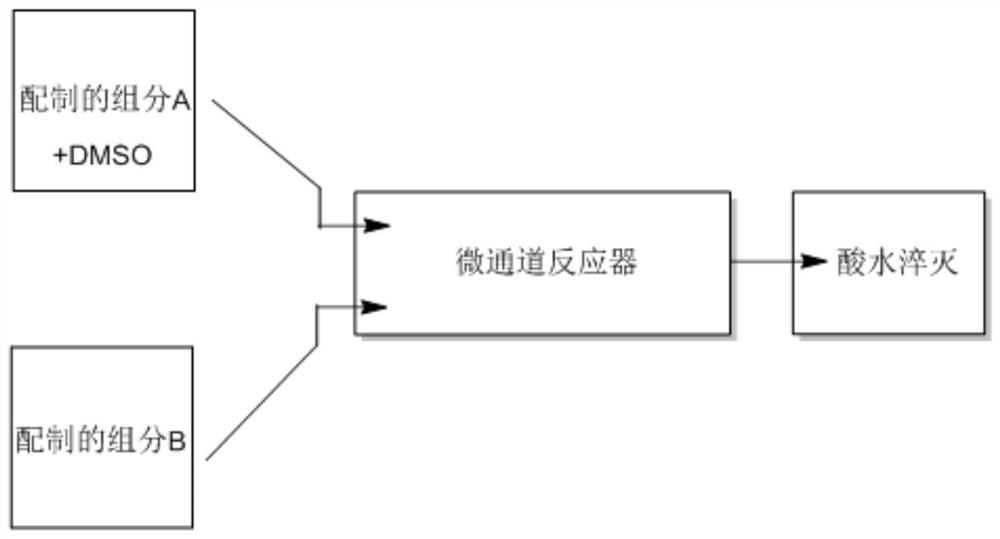
[0051] The method for synthesizing favipiravir using DMSO solvent in combination with microchannel reactor, the reaction flow is as follows figure 2 As shown, the reaction formula is as follows:
[0052]
[0053] Include the following steps:
[0054] 1. Prepare the solution:
[0055] Component A: 10g of 6-fluoro-3-hydroxypyrazine-2-carbonitrile (1eq) and 5.8g of NaOH (2eq) were added with 5.6g of DMSO (1eq), and 90ml of water was mixed into a clear solution (C=0.8mol / l);
[0056] Component B: 16.5g H 2 O 2 (2eq).
[0057] 2. Set the injection pump parameters:
[0058] Set the injection pump 1 (the injection pump 1 is connected with solution A) 0.5ml / min, 25 degrees Celsius, 0-1Mpa.
[0059] Set the injection pump 2 (the injection pump 2 is connected with solution B) 0.1ml / min, 25 degrees Celsius, 0-1Mpa.
[0060] 3. Set the microchannel reactor parameters:
[0061] 35 degrees Celsius, 0-1Mpa, 5 modules, heart-shaped structure.
[0062] Table 3. The reaction condi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com