Tissue engineering patch and preparation method thereof
A tissue engineering and patch technology, used in tissue regeneration, medical science, prosthesis, etc., can solve problems such as uneven distribution, cell damage, and sticking to the surface of scaffold materials, achieve high target cell load, increase tissue Compatibility, the effect of facilitating immigration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] This embodiment provides a method for preparing a tissue engineering patch, comprising the following steps:
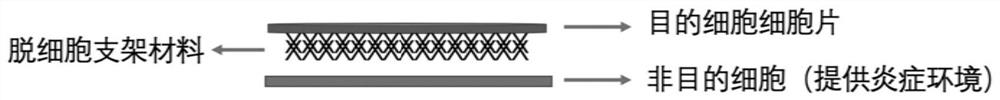
[0042] (1) Inoculate the target cells on one side of the decellularized scaffold, place pyroptotic products on the other side of the decellularized scaffold, and separate the pyroptotic products from the surface of the other side of the decellularized scaffold; The decellularized scaffold inoculated with the target cells and the pyroptosis product are cultured together in the culture medium;
[0043] (2) When the target cells migrate from one side of the decellularized scaffold to the other side, remove the non-migrated target cells on the surface of the decellularized scaffold to obtain a tissue engineering patch;
[0044] The pyroptotic product is at least one of pyroptotic secretion, pyroptotic extract and pyroptotic cells produced after pyroptosis.
[0045] The pyroptosis of the present invention refers to programmed cell inflammatory necrosis, which is cha...
Embodiment 1
[0060] Example 1 Construction of tissue engineered adipose tissue in vitro
[0061] (1) Pre-cultivate non-target cells to obtain an inflammatory environment
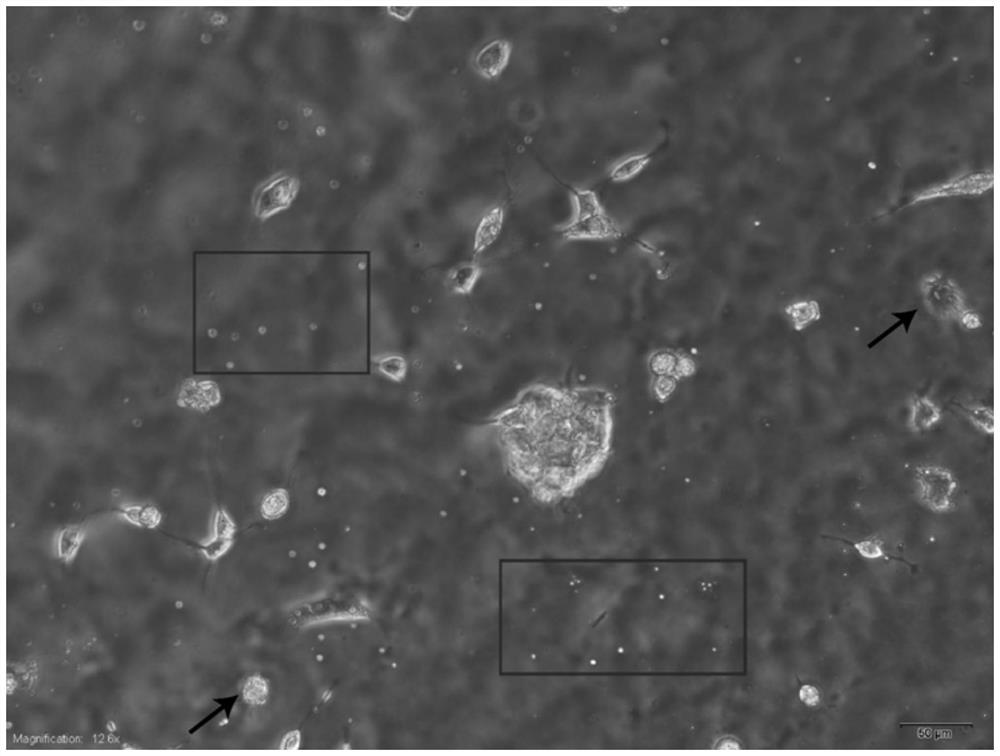
[0062] Bone marrow mesenchymal stem cells were used as non-target cells at 1*106cell / cm 2 The density was inoculated on a large dish with a diameter of 10 cm, and cultured in a carbon dioxide incubator for 24 hours. The medium was a complete medium: low-sugar DMEM+10% fetal bovine serum+1% glutamine+1% penicillin-streptomycin ( 100X). After 24 hours, replace the medium with a complete medium containing 2 mol / ml lipopolysaccharide, and culture for 36 hours. After bone marrow mesenchymal stem cells form obvious pyroptotic bodies (such as figure 2 As shown, the spherical object framed by the red box is the pyroptotic body, and the black arrow points to the pyroptotic cell), indicating that the culture of bone marrow mesenchymal stem cells induced pyroptosis is completed. The medium was removed, and the bottom of the dis...
Embodiment 2
[0077] Example 2: Construction of tissue engineered liver tissue in vitro
[0078] (1) Perform pyroptotic pre-stimulation on non-target cells to obtain an inflammatory environment.
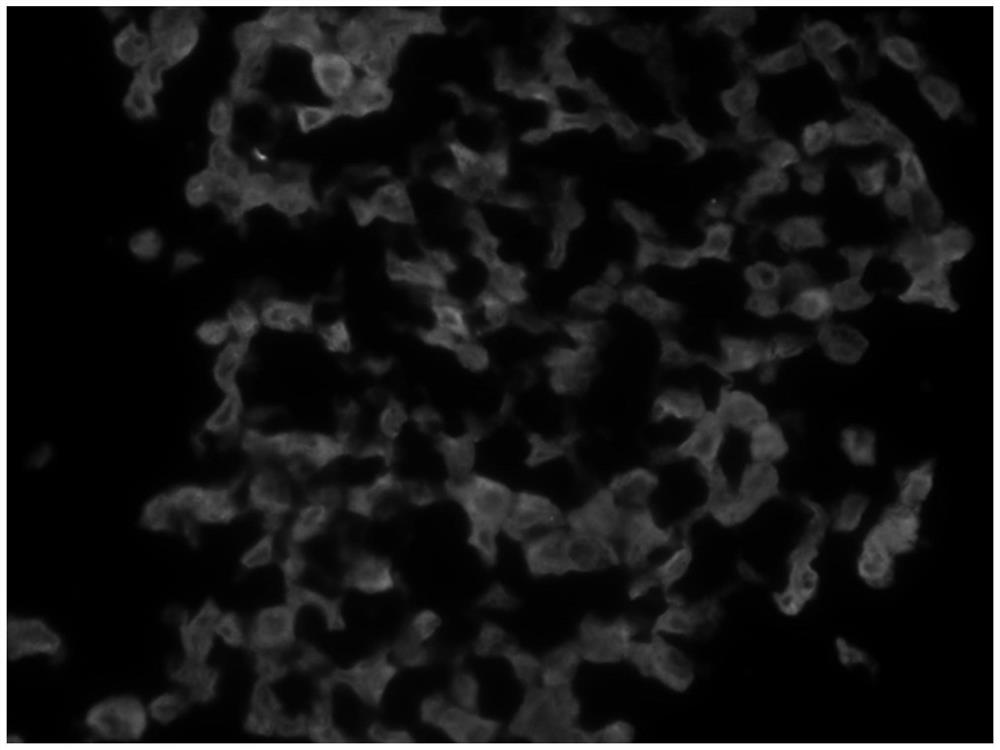
[0079] Isolate cardiosphere stem cells (CDCs), use cardiosphere stem cells as non-target cells, and use 5*106cell / cm 2 The density was inoculated on OPC (hydroxypropyl cellulose octanoate)-coated large dishes and cultured for 3 days. Three days later, the secretion of pyroptotic inflammatory factors increased, pyroptotic bodies were formed, and the pyroptotic marker caspase-1 was stained (such as image 3 ), indicating that the pyroptotic induction culture of cardiomyocyte stem cells was completed, and a large dish containing pyroptotic cardiosphere stem cells was obtained.
[0080] (2) Obtain a single-layer or multi-layer cell sheet of the target cells, and inoculate the obtained cell sheet on one side of the decellularized scaffold.
[0081] After suspending bone marrow mesenchymal stem cells...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com