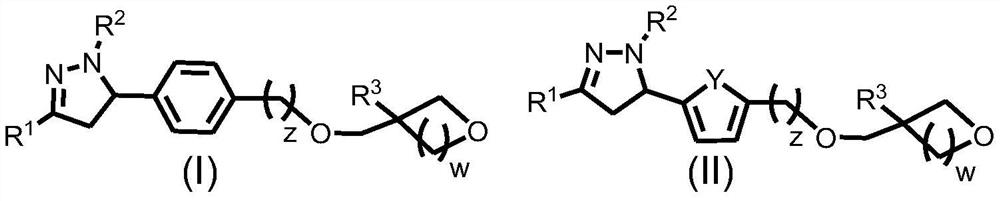
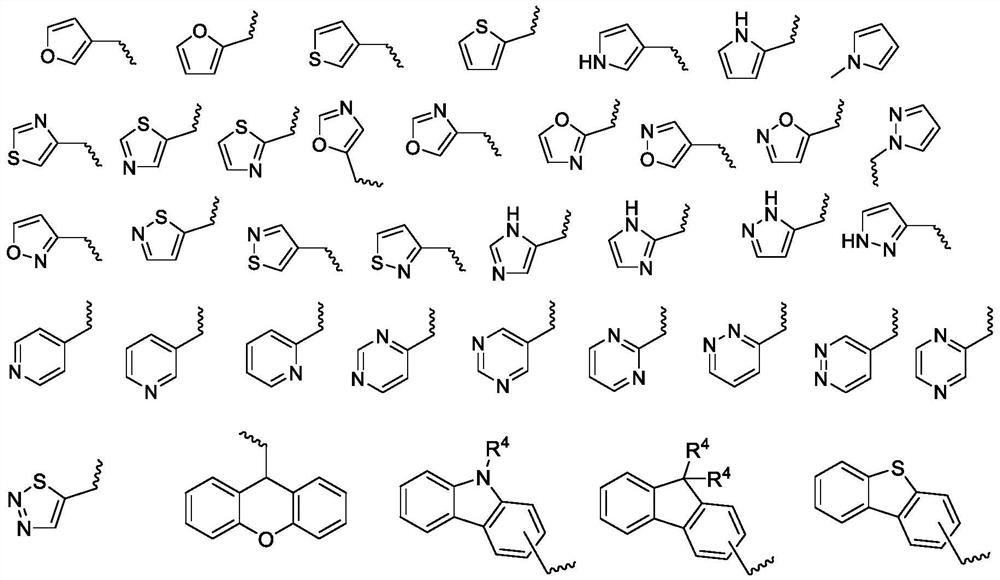
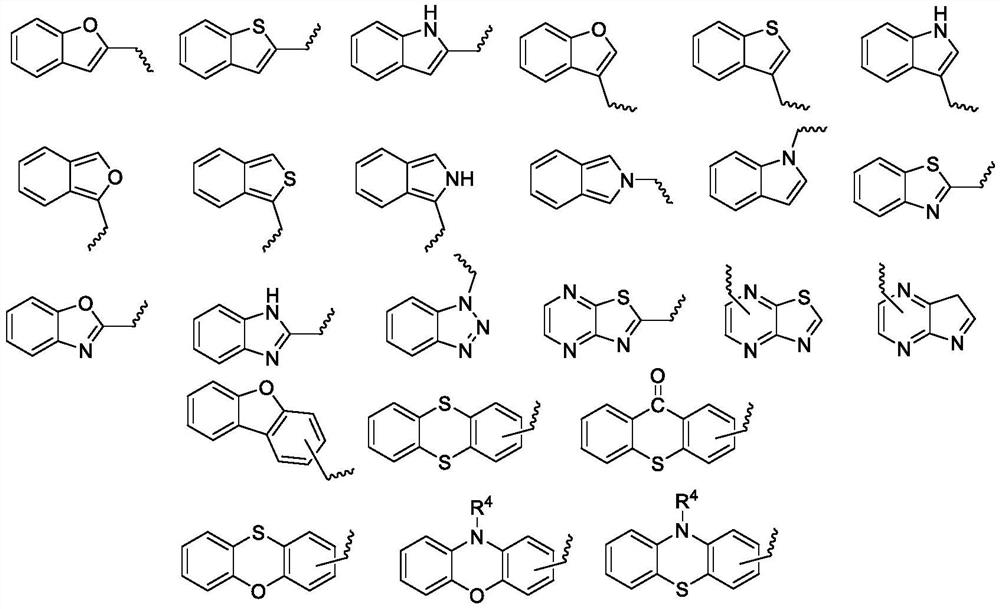
Epoxide-substituted pyrazoline derivative, photocurable composition and preparation method
A technology of epoxy and pyrazoline, applied in epoxy resin coatings, organic chemistry, coatings, etc., can solve problems such as product performance degradation, easy migration, and small molecule residues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Embodiment 1: Synthesize target molecule (I)-1 according to the following route
[0089]
[0090] (a) Sodium hydroxide, absolute ethanol, room temperature, 1h;
[0091] (b) Sodium hydroxide, absolute ethanol, 80°C, 2h
[0092] (c) Potassium hydroxide, tetrabutylammonium bromide, toluene, 110°C, 4h;
[0093] 1. Synthesis of 3-(4-hydroxymethylphenyl)-1-phenyl-2-en-1-one
[0094] Add acetophenone (12.00 g, 0.10 mol), 4-hydroxymethylbenzaldehyde (13.62 g, 0.10 mol) and absolute ethanol (30 mL) into a 100 mL three-necked flask containing a magnetic rotor, and stir at room temperature. Then an aqueous solution of sodium hydroxide (12.00 g, 0.30 mol, 12 mL) was prepared and added dropwise to the reaction system through a constant pressure dropping funnel. The reaction process was monitored by a silica gel chromatographic plate. After the reaction was completed, it was filtered, and the filtrate was concentrated and then filtered. The solid obtained by two filtrations wa...
Embodiment 2
[0099] Example 2: Synthesis of Target Molecules (I)-2 to (I)-8
[0100] The preparation method of described pyrazoline type photosensitizer is the same as embodiment one, changes aryl ethyl ketone and R 4 can be achieved. The specific yield and mass spectrometric characterization results are as follows.
[0101]
Embodiment 3
[0102] Example 3: Synthesis of Target Molecules (I)-9 to (I)-14
[0103] The preparation method of described pyrazoline type photosensitizer is the same as embodiment one, change substituted phenylhydrazine and R 4 can be achieved. The specific yield and mass spectrometric characterization results are as follows.
[0104]
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com