Oral sustained-release preparation of diclofenac sodium and preparation method of oral sustained-release preparation
A technology for diclofenac sodium and sustained-release preparations, which is applied to the oral sustained-release preparations of diclofenac sodium and the field of preparation thereof, can solve the problems of many preparation steps, complicated processes, long coating time, etc. Simple process, good slow release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
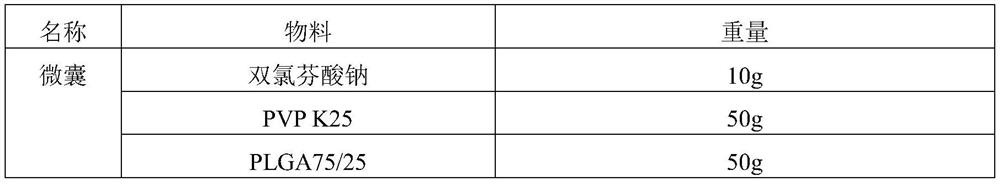
[0027] This embodiment provides an oral sustained-release preparation containing diclofenac sodium and a preparation method thereof, and the sustained-release preparation is obtained by the preparation steps of the prescriptions in Table 1 and Table 2:
[0028] Table 1
[0029]
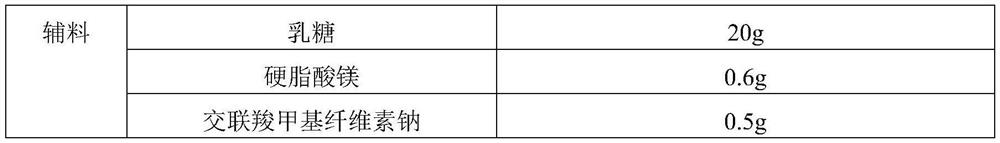
[0030] Table 2
[0031]
[0032]
[0033] Preparation Process:
[0034] Dissolve diclofenac sodium and PVP K25 in the inner layer solvent ethanol to obtain the inner layer solution; dissolve PLGA75 / 25 in the outer layer solvent acetone to obtain the outer layer solution; use the coaxial electrostatic spray method to separate the inner layer solution and the outer layer solvent Placed in a syringe, slowly output by a constant-current pump, sprayed out through a coaxial nozzle under the action of a high-voltage electric field, and collected by aluminum foil to obtain the diclofenac sodium microcapsules. Take an appropriate amount of microcapsules, add lactose, magnesium stearate, and croscarm...
Embodiment 2
[0036] This embodiment provides a kind of oral sustained-release preparation containing diclofenac sodium and its preparation method, and described sustained-release preparation is obtained by the prescription and preparation steps of Table 3 and Table 4:
[0037] table 3
[0038]
[0039] Table 4
[0040]
[0041] Preparation Process:
[0042] Dissolve diclofenac sodium and PVP K25 in the inner layer solvent ethanol to obtain the inner layer solution; dissolve PLGA75 / 25 in the outer layer solvent acetone to obtain the outer layer solution; use the coaxial electrostatic spray method to separate the inner layer solution and the outer layer solvent Placed in a syringe, slowly output by a constant-current pump, sprayed out through a coaxial nozzle under the action of a high-voltage electric field, and collected by aluminum foil to obtain the diclofenac sodium microcapsules. Take an appropriate amount of microcapsules, add microcrystalline cellulose, magnesium stearate, an...
Embodiment 3
[0044] This embodiment provides an oral sustained-release preparation containing diclofenac sodium and a preparation method thereof, and the sustained-release preparation is obtained by the preparation steps of the prescriptions in Table 5 and Table 6:
[0045] table 5
[0046]
[0047] Table 6
[0048]
[0049] Preparation Process:
[0050] Dissolve diclofenac sodium and PVP K25 in the inner layer solvent ethanol to obtain the inner layer solution; dissolve PLGA75 / 25 in the outer layer solvent acetone to obtain the outer layer solution; use the coaxial electrostatic spray method to separate the inner layer solution and the outer layer solvent Placed in a syringe, slowly output by a constant-current pump, sprayed out through a coaxial nozzle under the action of a high-voltage electric field, and collected by aluminum foil to obtain the diclofenac sodium microcapsules. Take an appropriate amount of microcapsules, add lactose, microcrystalline cellulose, magnesium steara...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com