Lactobacillus mucoase for relieving endotoxin infection and application
A technology of Lactobacillus mucosae and endotoxin, applied in the field of microorganisms, can solve the problems of insignificant curative effect, inability to effectively remove endotoxin, insufficient clinical data, etc., and achieve the effect of increasing the content of short-chain fatty acids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Screening, identification, cultivation and preservation of mucosal lactobacillus CCFM1142
[0049] 1. Screening
[0050]Take 1g of healthy human feces samples from Mayang Miao Autonomous County, Huaihua City, Hunan Province, apply them on MRS solid medium after gradient dilution, and culture them in an anaerobic environment at 37°C for 72 hours, observe and record the colony morphology; , Protruding, white and yellowish colonies were streaked on the MRS solid medium, and purified and cultivated under anaerobic conditions at 37°C. Repeat this operation 3 times to obtain purified single colonies; pick a single colony on Stretch on the MRS solid medium, culture anaerobically at 37°C for 36 hours, perform Gram staining on the obtained colonies, record the morphology of the colonies, and investigate the physiological and biochemical characteristics of the strains according to the "Common Bacterial System Identification Manual", and retain Gram-negative , the colo...
Embodiment 2
[0065] Embodiment 2: Preparation of Lactobacillus mucous membrane CCFM1142 bacterium liquid
[0066] (1) Dip the bacterial solution of Lactobacillus mucous membranes CCFM1142 from the glycerol tube, streak it on the MRS solid medium, and culture it at 37°C for 48 hours under anaerobic environment to obtain a single colony; pick a single colony and inoculate it in the MRS liquid medium, In an anaerobic environment, culture at 37° C. for 48 hours for activation culture, and repeat this operation 3 times to obtain the activated bacterial liquid.
[0067] (2) Inoculate the activated bacterial liquid obtained in step (1) into the MRS liquid medium according to the inoculum size of 2% (v / v), cultivate at 37°C for 24 hours to obtain a fermentation liquid, and collect the bacteria by centrifugation of the fermentation liquid body, resuspend the cell body with physiological saline, and adjust the number of viable cells to 5×10 9 CFU / mL, made into bacterial suspension.
Embodiment 3
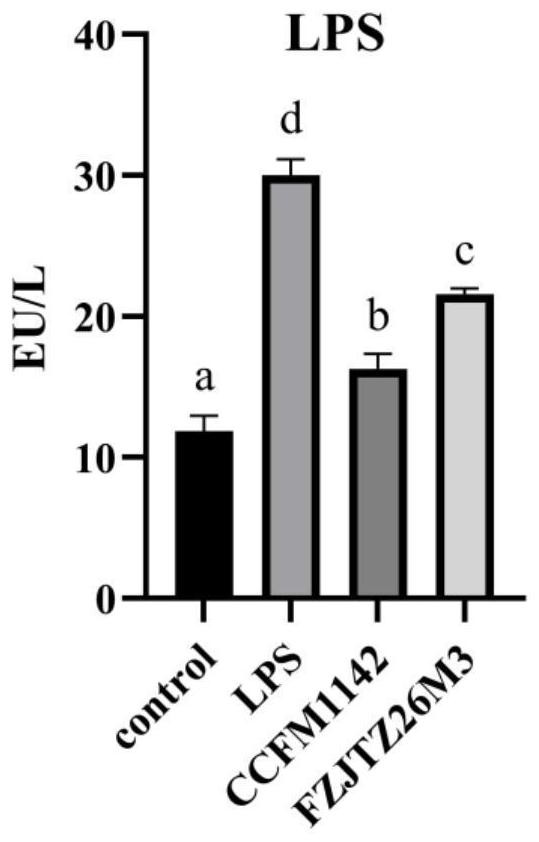
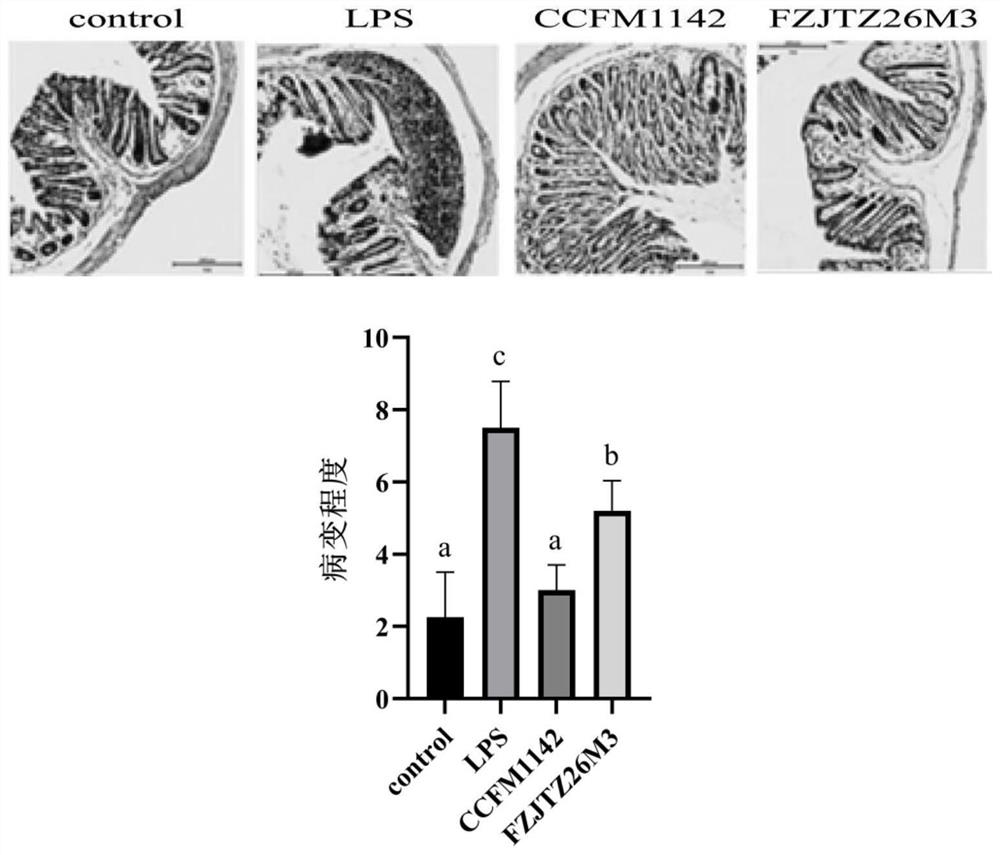
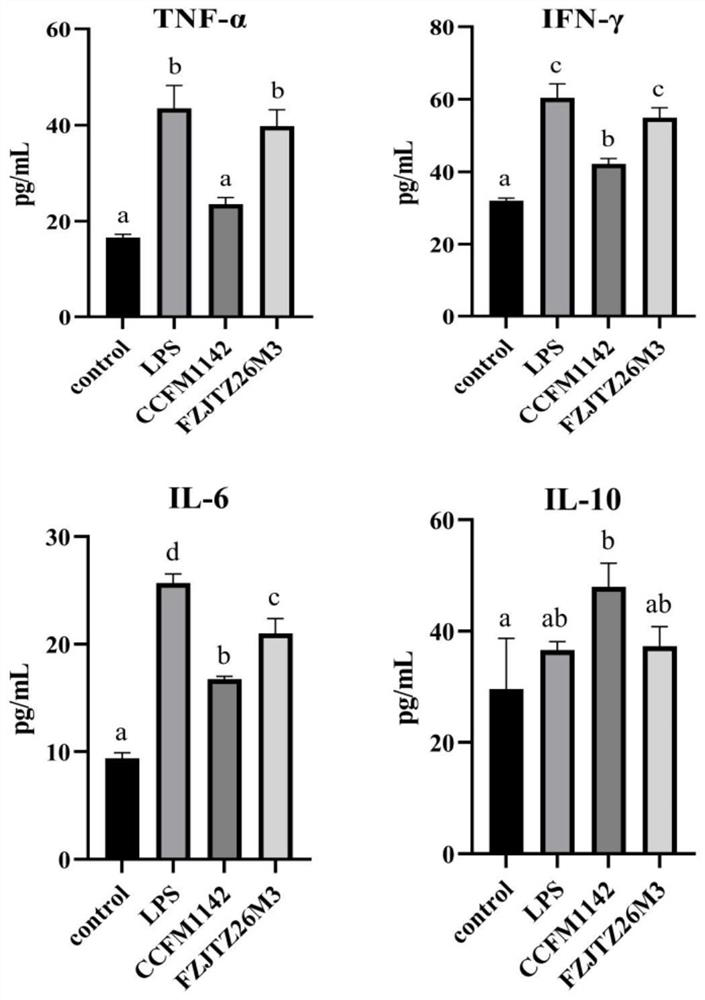
[0068] Example 3: Effect of Lactobacillus CCFM1142 on LPS Infection of LPS in Mouse Serum LPS
[0069] Fifty-six 6-week-old, 18-20g C57BL / 6J male mice were randomly divided into 4 groups after a one-week adaptation period: control group, LPS model group, CCFM1142 group and FZJTZ26M3 group, with 8 mice in each group. Among them, the control group was intraperitoneally injected with 0.2mL of normal saline and gavaged with 0.2mL of protective agent every day (the preparation method of the protective agent was: 130g / L skim milk was sterilized at 105°C for 10min; 20g / L of sucrose solution was mixed with 20g / L trehalose solution was autoclaved at 115°C for 20 minutes; the three solutions were mixed at a volume ratio of 1:1:1 to be the protective agent); the LPS modeling group was intraperitoneally injected with 0.2 mL of LPS solution (LPS dissolved in LPS solution with a final concentration of 0.15 mg / kg was made from normal saline) and 0.2 mL of protective agent was administered i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com