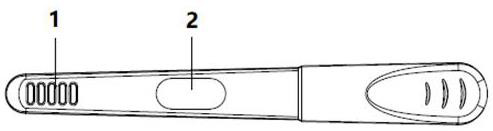
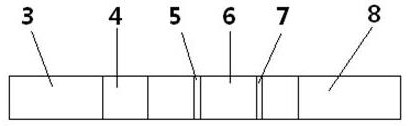
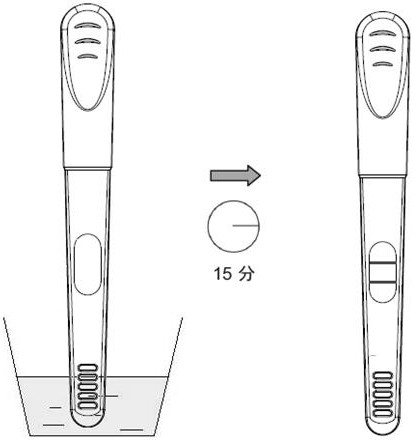
Urine helicobacter pylori antibody detection kit and preparation method thereof
A technology for Helicobacter pylori and antibody detection, which is applied in biological testing, measuring devices, immunoassays, etc., can solve problems such as low user acceptance, unsuitable home testing, and inability to detect past infections, and achieves convenient sampling and improved sensitivity. , the effect of high detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061]Example 1 Preparation and use of the kit of the present invention
[0062]a. Time-resolution fluorescent microsphere mark: Take 100 ul time to resolve fluorescent microspheres, dilute to 1 ml with ultrapure water. Take 40 ul of 50 mg / ml NHS (N-hydroxy succinimide) solution to mix well in the microsphere suspension, then add 40 uL of 50 mg / ml EDC (1- (3-dimethylaminopropyl) -3- Ethyl carbon diimide hydrochloride solution is mixed in a microsphere suspension, and the reaction is 0.5 hours at room temperature. The reaction after the reaction was resuspended with ultrasonic ultrasound, and then the microsphere suspension was centrifuged, sucks the supernatant, and 1 ml of ultra-pure water was added, and then uniformly dispersed evenly, and the activation of the fluorescent microspheres was completed. Take 1 ml of activated microsphere suspension, ultrasonic dispersion is uniform, then dripped with antigens antibody while stirring, the marking amount is 0.01 mg-0.05 mg / ml, prefe...
Embodiment 2
[0085]Example 2: Sensitivity, Specific Verification Comparative Experiment:
[0086]Sample: Take the blood and urine samples of one person, collect 50 samples, including 29 cases of positive cases of Helicobacter, and 22 cases of negative cases. The blood sample was synchronized with the urine sample with the urine sample with the following different detection methods.
[0087]Control group 1: Double antigen sandwich gum gold method: Gold bonding pad contains Helicobacteri antigen A-labeled colloidal gold, nitrocellulose film is packed by Helicobacterium antigen B
[0088]Control group 2: Double antigen sandwich method and indirect method combined with colloidal gold method: Gold bonding pad contains Helicobacter antigen A-labeled colloidal gold and anti-human IgG labeled colloidal gold mixture, nitrocellulose film is packed by H. pylori antigen B
[0089]Control Group 3: Double antigen sandwich method and indirect method combined with fluorescent microspheres: fluorescent pad containing a prac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com