Preparation and application of escherichia coli preferred soluble porcine PD-1 recombinant protein
A PD-1, recombinant protein technology, applied in the field of biomedicine, can solve problems such as unsatisfactory effects, and achieve the effect of improving immunity and enhancing immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The preparation method of embodiment 1 soluble porcine PD-1 recombinant protein
[0040] (1) Escherichia coli codon transformation of porcine PD-1 protein extracellular region gene
[0041] Follow the principle of E. coli codon partial tropism table, the G+C content of the gene sequence should not be higher than 60%, and respect the original codon of the gene to transform the gene of the extracellular region of the porcine PD-1 protein, and also carry out the gene sequence primer design The modification of E. coli codon bias tropism, plus the specific primer sequence of restriction site is shown in Table 1. The major amino acid sites were modified as follows: ACA→ACC, GAG→GAA, CCC→CCG, CTC→CTG, CGC→CGT, AGG→CGT. The nucleotide sequence of the DNA molecule of the obtained Escherichia coli partial tropism soluble porcine PD-1 protein is shown in SEQ ID NO:2. Send the designed and modified gene sequence to Sangon Bioengineering (Shanghai) Co., Ltd. for synthesis.
[004...
Embodiment 2
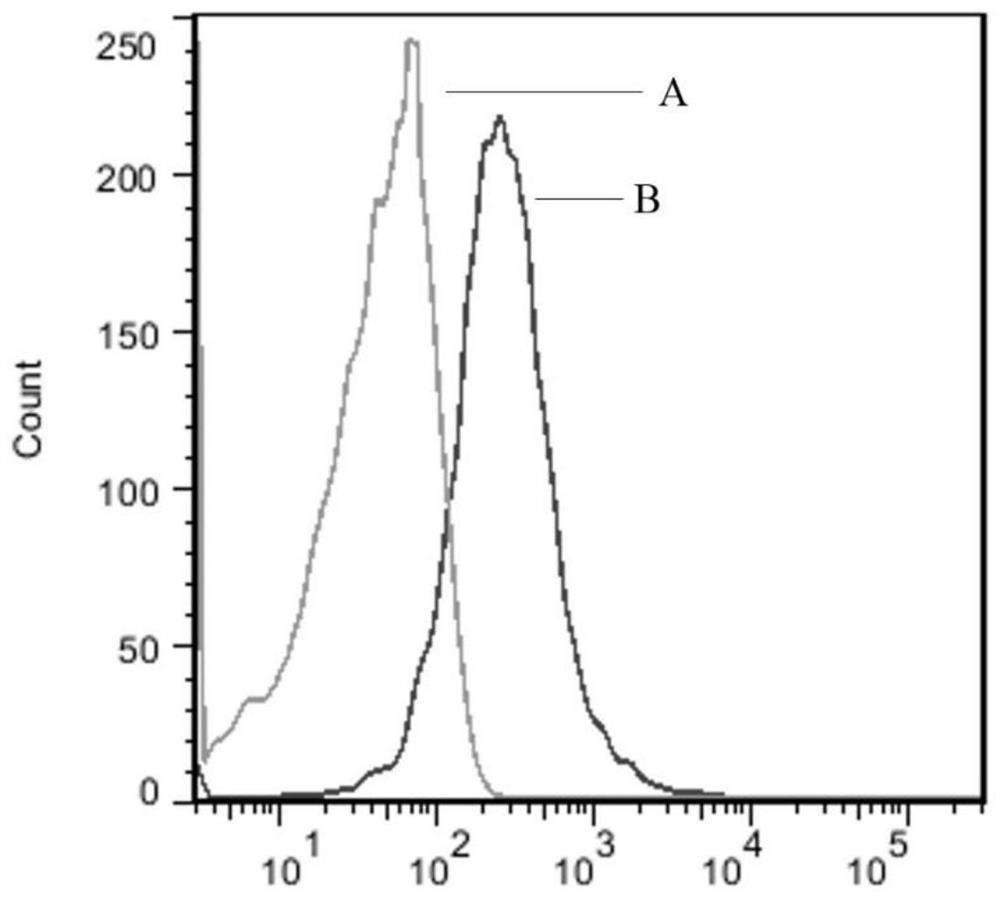
[0050] Example 2 In vitro activity identification method of soluble porcine PD-1 recombinant protein as a vaccine enhancer
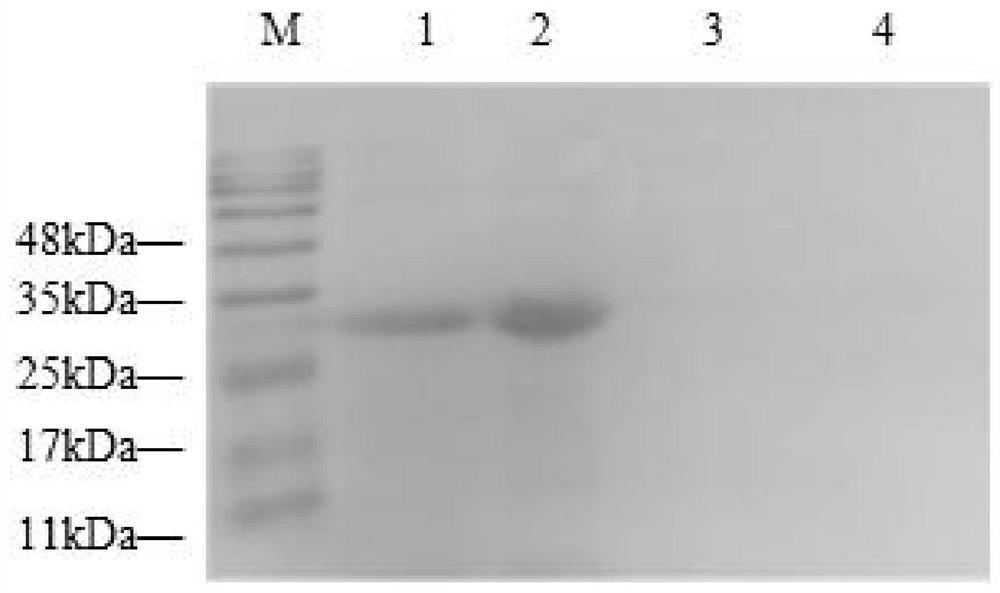
[0051] (1) Western blotting (Western blotting) to identify PD-1 recombinant protein activity
[0052] Western blotting to identify the induced expression of soluble porcine PD-1 recombinant protein, the specific steps are as follows:
[0053] 1) Gel preparation: First, add each component in the beaker according to the system in Table 2 to prepare a 12% separating gel. After the gel is solidified, use filter paper to absorb excess water in the glass plate, and then prepare the concentrated gel according to Table 3.
[0054] Table 2 Preparation of 12% separating gel
[0055] components volume sterile water 4.9mL 30% acrylamide 6.0mL 1.5mM Tris-HCl buffer 3.8mL 10% Sodium Lauryl Sulfate 150.0μL 10% ammonium persulfate 150.0μL Tetramethylethylenediamine 6.0 μL
[0056] Table 3 Preparation of 5% stac...
Embodiment 3
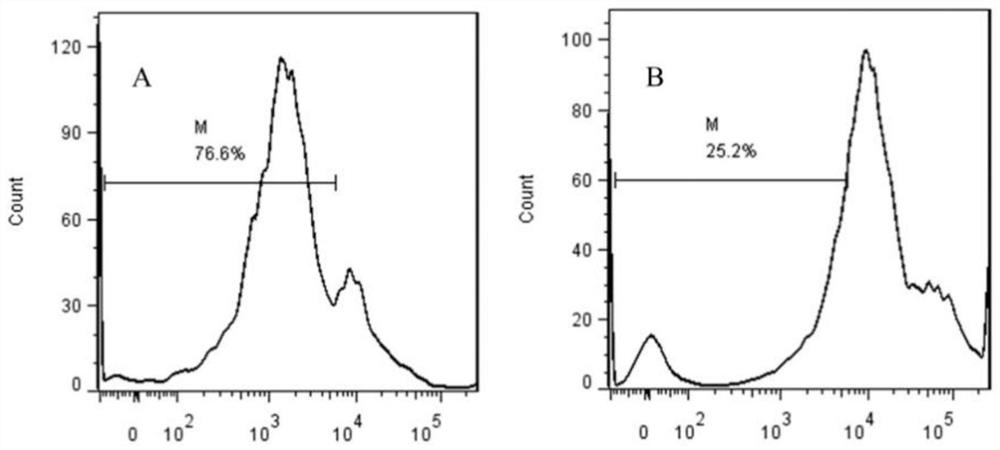
[0087] Example 3 In vivo activity identification method of soluble porcine PD-1 recombinant protein as a vaccine enhancer
[0088] (1) Animal grouping
[0089] By consulting the literature and equivalent dose conversion among animals, it was determined that the immunization dose of the soluble porcine PD-1 recombinant protein was 0.2 mg / kg. Select 40 weaned piglets that were not immunized with PCV2 vaccine at the age of 45 days, and randomly divide them into 4 groups, 10 pigs in each group. Group A is the PCV2 vaccine immunization group, groups B and C are the test groups, and group D is the control group for irrelevant proteins , and the specific grouping conditions are shown in Table 7.
[0090] Table 7 Experimental animal groups and inoculation preparations
[0091] group number of animals Molecular preparation Vaccination method protein immunization dose A 10 heads PCV2 vaccine intramuscular injection 0mg / kg B 10 heads PCV2 vaccine +...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com