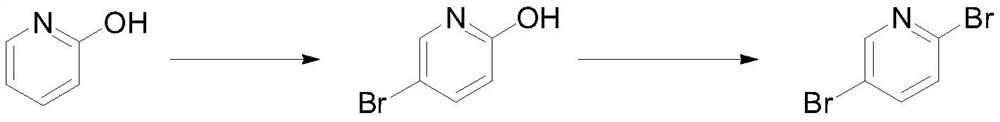
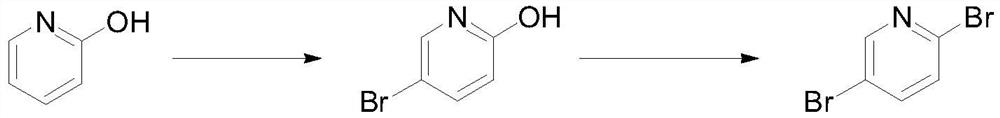
Preparation method of 2, 5-dibromopyridine
A technology of dibromopyridine and hydroxypyridine, which is applied in the field of preparation of 2,5-dibromopyridine, can solve the problems of easy decomposition and explosion, high activity, and large amount of waste water, and achieve reduced waste water production, high regioselectivity, and easy The effect of the operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022]
[0023] Put 50g (0.526mol) of 2-hydroxypyridine and 250mL of acetonitrile into the reaction bottle, control the temperature at -10-0°C and add 93.6g (0.526mol) of NBS in batches, after the addition, raise the temperature to 15-25°C to react 1 Hour. Sample HPLC was used to detect the completion of the reaction of the raw materials, and the ratio of the product 2-hydroxy-5-bromopyridine to the isomer 2-hydroxy-3-bromopyridine was 94:6. Concentrate the reaction solution under reduced pressure at 45°C, raise the temperature of the remaining materials to 50°C, dissolve the materials, measure the pH=6.0-7.0, add 750mL of water to control the temperature at 45-50°C, and slowly cool down to 20-25°C, the materials precipitate and Stir at temperature for 3 hours and filter. The filter cake was rinsed with water and petroleum ether, and dried to obtain 82.6 g of 2-hydroxy-5-bromopyridine, with a yield of 90.3%, HPLC: 95.6%. 1 HNMR (400MHz, CDCl 3 ):11.73(s,1H),7.70(d,1H),7....
Embodiment 2
[0025]
[0026] Put 50g (0.526mol) of 2-hydroxypyridine and 200mL of 1,2-dichloroethane into the reaction flask, and add 90g (0.315mol) of dibromohydantoin in batches under control of the temperature at -10-0°C. After the addition is complete, Raise the temperature to 20-25°C and react for 1.5 hours. Sample HPLC was used to detect the completion of the reaction of the raw materials, and the ratio of the product 2-hydroxy-5-bromopyridine to the isomer 2-hydroxy-3-bromopyridine was 93:7. Add sodium bisulfite aqueous solution to quench, separate layers, extract the aqueous phase with 1,2-dichloroethane, combine the organic phases, wash with water to pH = 6.0-7.0, dry the organic phase with anhydrous sodium sulfate to obtain 2- Hydroxy-5-bromopyridine in 1,2-dichloroethane solution 435g, HPLC: 92.6%, yield 92.0%; to be brominated in the next step.
Embodiment 3
[0028]
[0029] Under nitrogen protection, drop 50g (0.287mol) 2-hydroxyl-5-bromopyridine, 7.3g (0.014mol) tris (pentafluorophenyl) borane and 200mL acetonitrile into the reaction flask, drop 93.2g ( 0.344mol) phosphorus tribromide, slowly warming up to reflux reaction for 5 hours after the dropwise addition. Detect that the reaction of the raw materials is complete, concentrate under reduced pressure at 55°C until no liquid flows, pour the remaining liquid into 200mL of ice water, add saturated sodium carbonate solution to adjust the pH=8.0-9.0, extract with dichloromethane, concentrate the solvent under reduced pressure, add 35mL of iso Propanol was replaced, the temperature was raised to 50°C, 100 mL of water was added, the temperature was slowly lowered to precipitate a solid, filtered, and the filter cake was dried to obtain 56.3 g of 2,5-dibromopyridine, with a yield of 82.7%, HPLC: 99.7%. 1 HNMR (400MHz, CDCl 3 ): 8.81(d,1H), 8.35(d,1H), 8.13(d,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com