Amphiphilic conjugated oligomer, preparation thereof and drug-loaded nanoparticles prepared by self-assembly of amphiphilic conjugated oligomer
A technology of nanoparticles and oligomers, applied in the field of materials, can solve problems such as long cycle time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
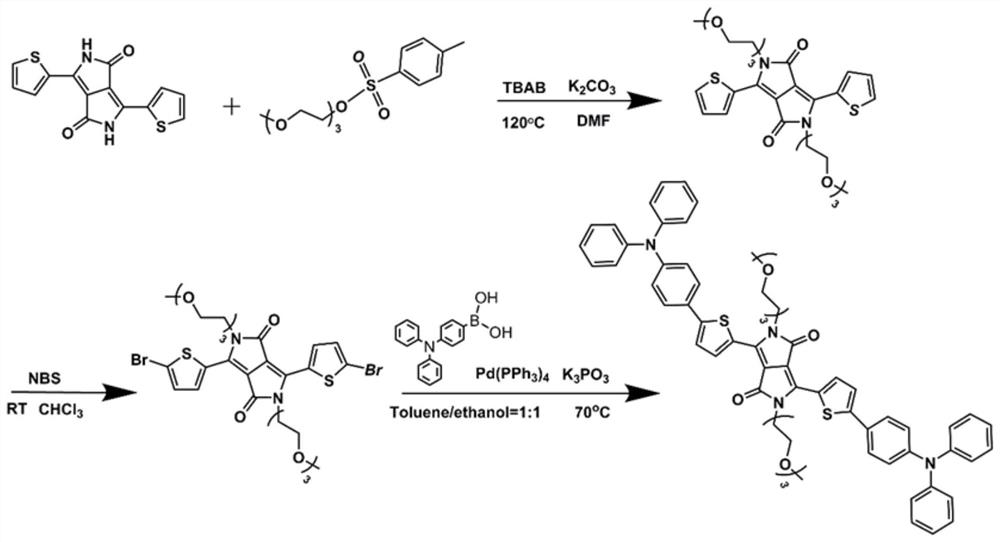
[0076] Example 1, conjugated oligomer 3,6-bis(5-(4-(2-(diphenylamino)phenyl)thiophen-2-yl)-2,5-bis(2-(2-( 2-(2-Methoxyethoxy)ethoxy)-ethyl)-2,5-dihydropyrrole[3,4-c]pyrrole-1,4-dione (DPP-TEG-TPA) Synthesis
[0077] according to figure 1 The synthetic route shown prepares 3,6-bis(5-(4-(2-(diphenylamino)phenyl)thiophen-2-yl)-2,5-bis(2-(2-(2-( 2-Methoxyethoxy)ethoxy)-ethyl)-2,5-dihydropyrrole[3,4-c]pyrrole-1,4-dione (DPP-TEG-TPA)
[0078] 1) Synthesis of 3,6-di(thiophen-2-yl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione
[0079] Add 90 mL of tert-amyl alcohol into a 500 mL two-necked bottle, and add sodium tert-amyl alcohol (24.8 g, 225 mmol) under an argon atmosphere. Raise the temperature to 90°C and stir until the sodium tert-amylate is completely dissolved. After adding 2-cyanothiophene (16.4 g, 150 mmol) dropwise into the system, diisopropyl succinate (12.1 g, 60 mmol) was slowly added dropwise, and it was observed that the color of the suspension changed to deep red. After ...
Embodiment 2
[0091] Example 2, Preparation of Oligomer Self-Assembled Nanoparticles DPP-TEG-TPA NPs
[0092] 1) Weigh DPP-TEG-TPA (1mg) and dissolve it in 1mLTHF, sonicate for 20min;
[0093] 2) Add the mixture to 5mL ultrapure water, and sonicate for 10min;
[0094] 3) Bubble an inert gas into the solution and stir slowly for 1 hour;
[0095] 4) After passing through a 220 μm filter membrane, dialyze overnight in a 3500 KDa dialysis bag at room temperature to remove free molecules, collect the samples in the dialysis bag, and store them at 4°C for later use.
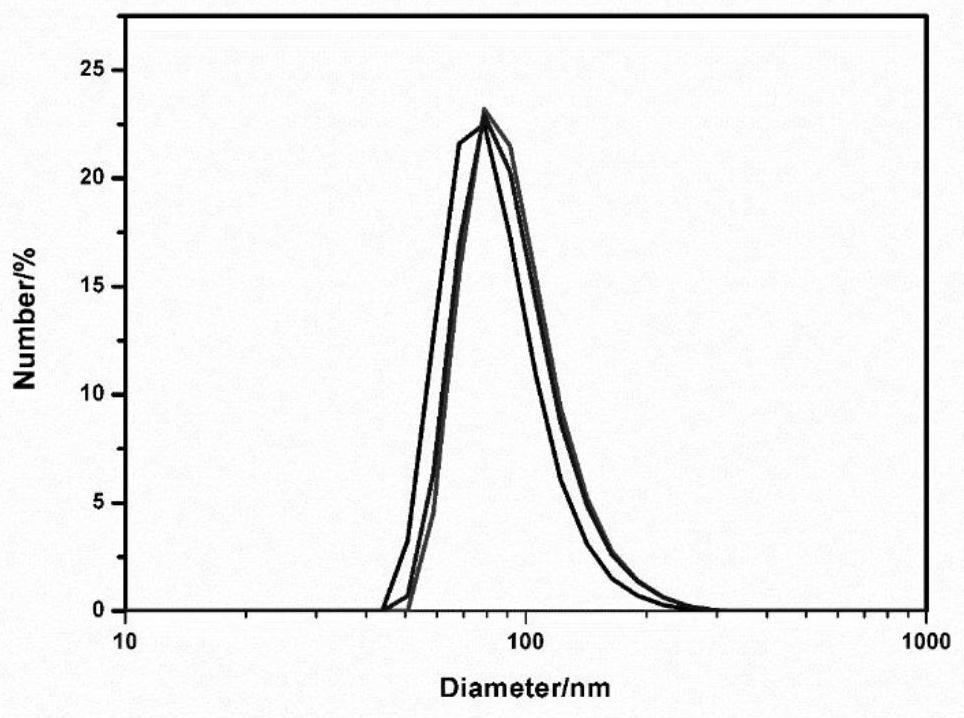
[0096] image 3 The particle size characterization of the prepared nanoparticles DPP-TEG-TPA NPs.
[0097] Depend on image 3 It can be seen that the particle size of the nanoparticles measured by the dynamic light scattering method is about 100 nm, and the particle size is uniform.
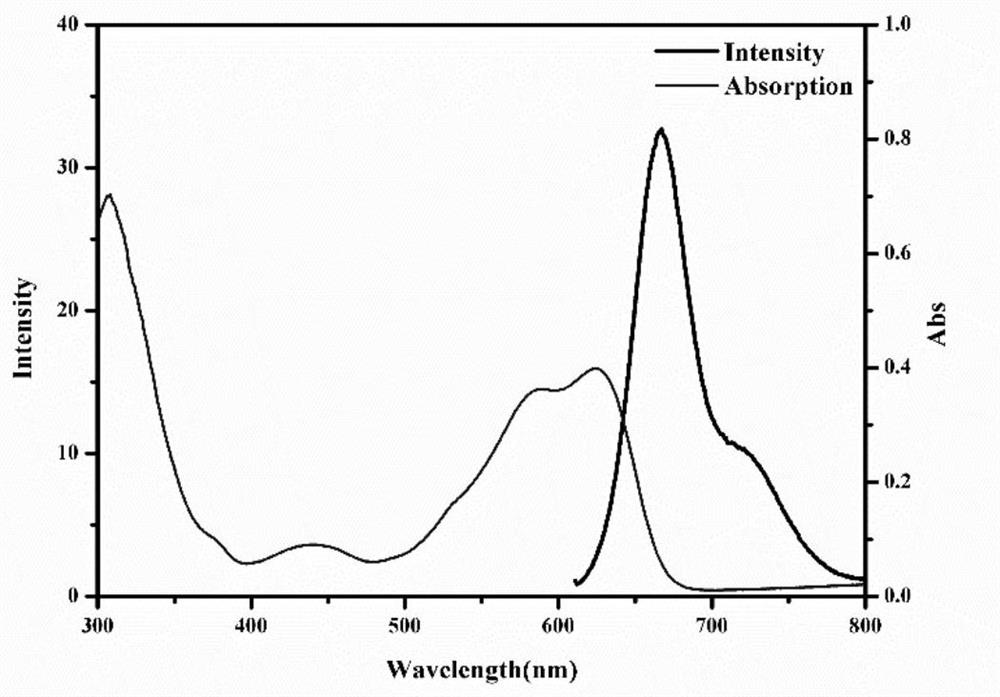
[0098] Figure 4 The ultraviolet-visible absorption spectrum characterization of the prepared nanoparticles DPP-TEG-TPA NPs.
[0099] Depend on...
Embodiment 3
[0102] Example 3, preparation of loaded curcumin nanoparticles DPP-TEG-TPA@Cur NPs
[0103] 1) Dissolve DPP-TEG-TPA (1mg) in 1mL THF and curcumin (0.3mg) in 0.3mL THF, sonicate for 20min respectively;
[0104] 2) Heat 6mL ultrapure water bath to 30°C, mix the above two solutions, add into ultrapure water, and sonicate for 10min;
[0105] 3) Bubble an inert gas into the solution and stir slowly for 1 hour;
[0106] 4) After passing through a 220 μm filter membrane, dialyze overnight in a 3500KDa dialysis bag at room temperature to remove free molecules, collect samples in the dialysis bag, perform ultrafiltration three times to remove unloaded curcumin molecules, and store the obtained filtrate at 4°C;
[0107] Image 6 Particle size characterization of the prepared drug-loaded nanoparticles DPP-TEG-TPA@Cur NPs
[0108] Depend on Image 6 It can be seen that the particle size of the nanoparticles DPP-TEG-TPA@Cur NPs measured by the dynamic light scattering method is about 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com