Polypeptide and application thereof in preparation of ACE inhibitor or blood pressure reducing product
An inhibitor and blood pressure lowering technology, applied in the field of biomedicine, can solve problems such as unsuitable for industrial application and complex structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
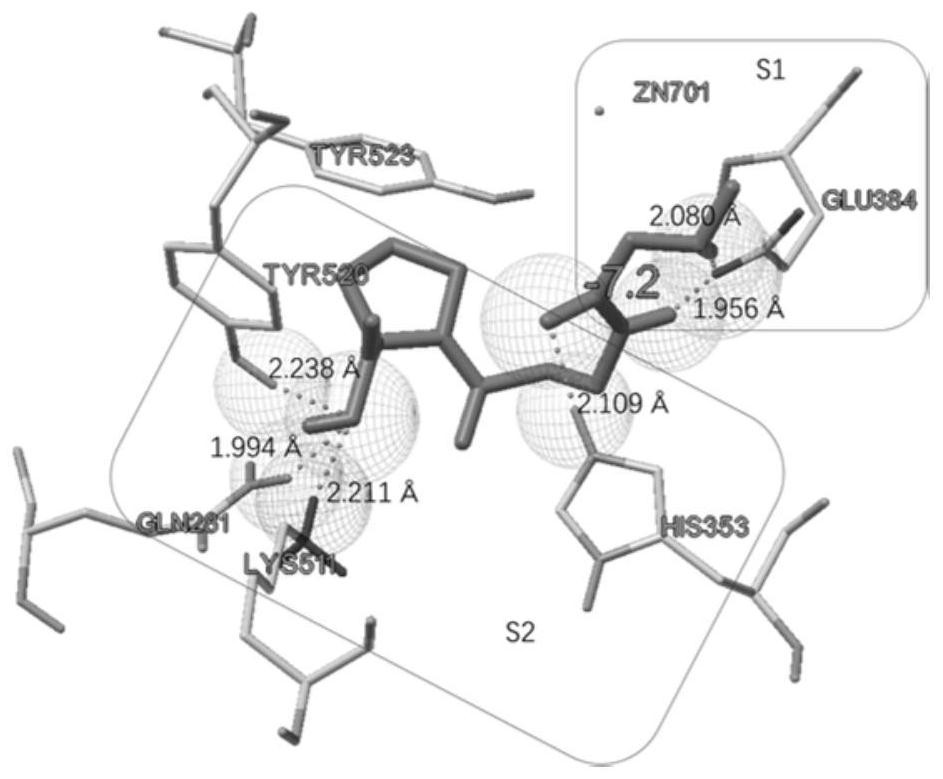
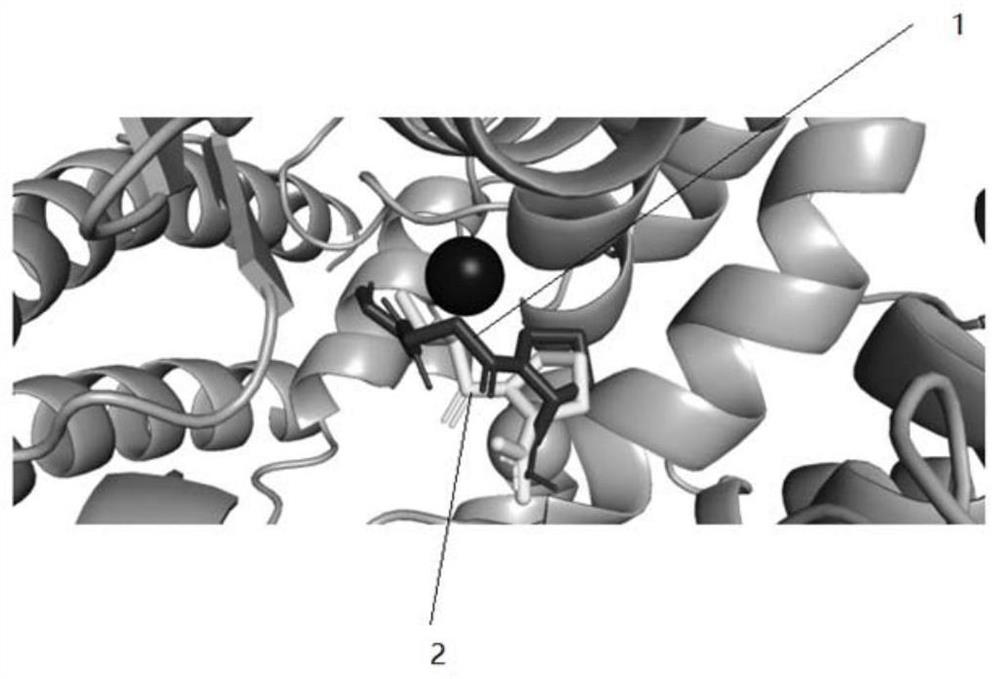
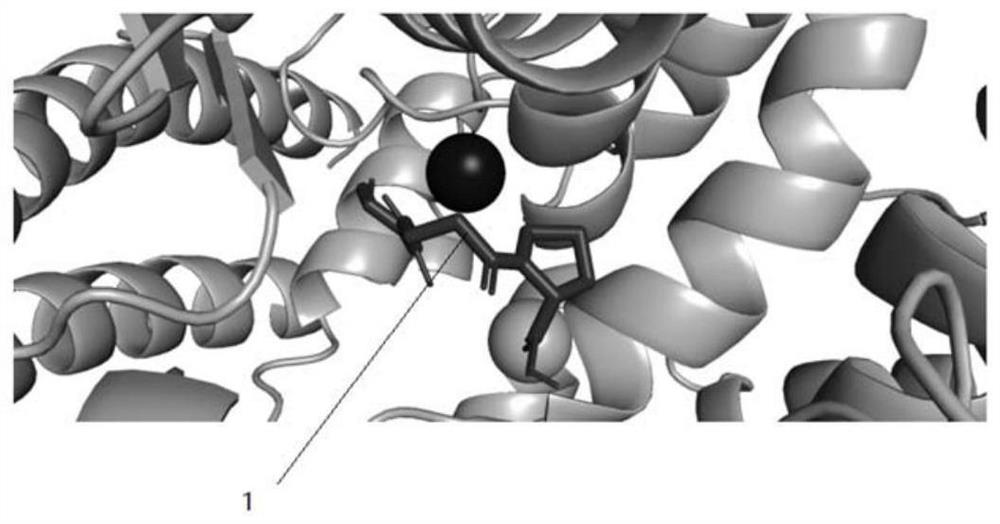
[0031] Example 1 Molecular docking analysis of the combination of polypeptide compound Sar-bAla-Pro and ACE
[0032] First, the structure of the polypeptide compound Sar-bAla-Pro was generated by chemdraw software
[0033] And the crystal structure of human ACE (1o86.pdb) was extracted from the protein database. Then, the ACE protein (including its cofactors chloride and zinc) in PDBQT format was extracted using the AutoDock tool software (v1.5.6), and Gasteiger charges were assigned and polar hydrogen bonds were added.
[0034] Subsequently, molecular docking simulations were performed using AutoDock Vina software (v1.1.2) to predict the binding affinity and conformation of compounds to ACE. ACE was set to rigid throughout the simulation. Scoring uses a weighted sum of steric and hydrophobic interactions, as well as hydrogen bonds. When running molecular docking, the exhaustiveness parameter was set to 32 and the coordinates were set to X:40.69, Y:32.80, and Z:47.29. Th...
Embodiment 2
[0037] Synthesis of embodiment 2 polypeptide compound Sar-bAla-Pro
[0038] The polypeptide compound Sar-bAla-Pro of the present invention is artificially synthesized, and the specific operation is as follows:
[0039] First, Fmoc-β-alanine was added to the proline dichloro resin, and TBTU (O-benzotriazole-N,N,N',N'-tetramethyluronium tetrafluoroboric acid and DIEA (N,N-diisopropylethylamine) for coupling, the coupling time is 60 minutes, and wash 3 times with piperidine / dimethylformamide with a volume ratio of 20% to remove the terminal Fmoc, and then use hexahydropyridine Carry out deprotection, and after deprotection, wash 6 times with piperidine / dimethylformamide whose volume ratio is 20%, and obtain an intermediate product after drying;
[0040] Add the intermediate product to Fmoc-sarcosine, TBTU and DIEA for coupling, the coupling time is 60 minutes, and wash 3 times with piperidine / dimethylformamide with a volume ratio of 20%, and deprotect with hexahydropyridine Aft...
Embodiment 3
[0043] ACE inhibitory activity detection of the polypeptide compound Sar-bAla-Pro of embodiment 3
[0044] Hippuryl-L-histidyl-L-leucine (hippuryl-L-histidyl-L-leucine, HHL) is rapidly decomposed under the catalysis of ACE enzyme to produce hippuric acid (Hippuric Acid, HA) and dipeptide histidyl-leucine (HL), adding After ACE enzyme inhibitor, the activity of ACE enzyme is inhibited, and the production of HA and HL is reduced. In this embodiment, ACE is extracted from rabbit lung, and the enzyme activity is 3.19mU / mL. It is developed by DAB and measured by spectrophotometer. The amount of HA produced and the activity of ACE enzyme were analyzed. Ethyl acetate extracts hippuric acid in the reactant, then reacts with a pyridine solution (DAB chromogen) containing p-dimethylaminobenzaldehyde in acetic anhydride to generate an orange-yellow compound, and directly measures its OD value at 459nm colorimetrically, The inhibition rate of ACE enzyme inhibitors to ACE enzyme was evalu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com