Preparation method of metaphosphate-coated lithium cobalt oxide material and lithium ion battery containing metaphosphate-coated lithium cobalt oxide material
A technology of metaphosphate-coated lithium cobalt oxide and phosphate-coated lithium cobalt oxide, applied in the field of functional material preparation, can solve the problems of high price of LiCoO, inability to meet performance requirements, slow growth of cobalt output, etc. The effect of discharge capacity and cycle stability, easy operation, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
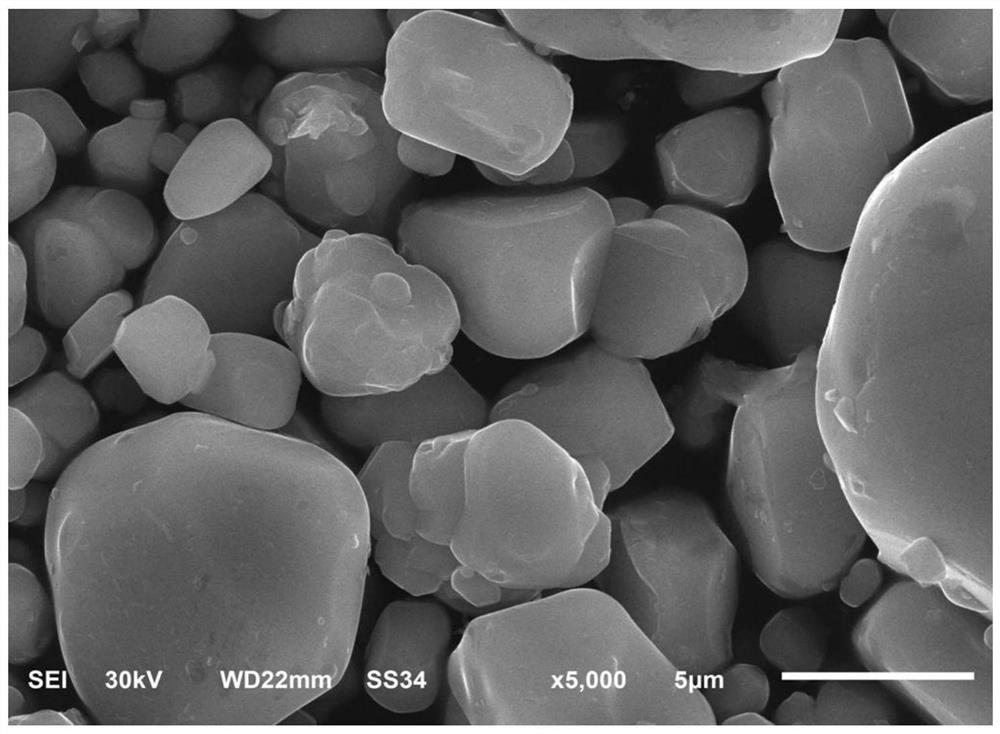
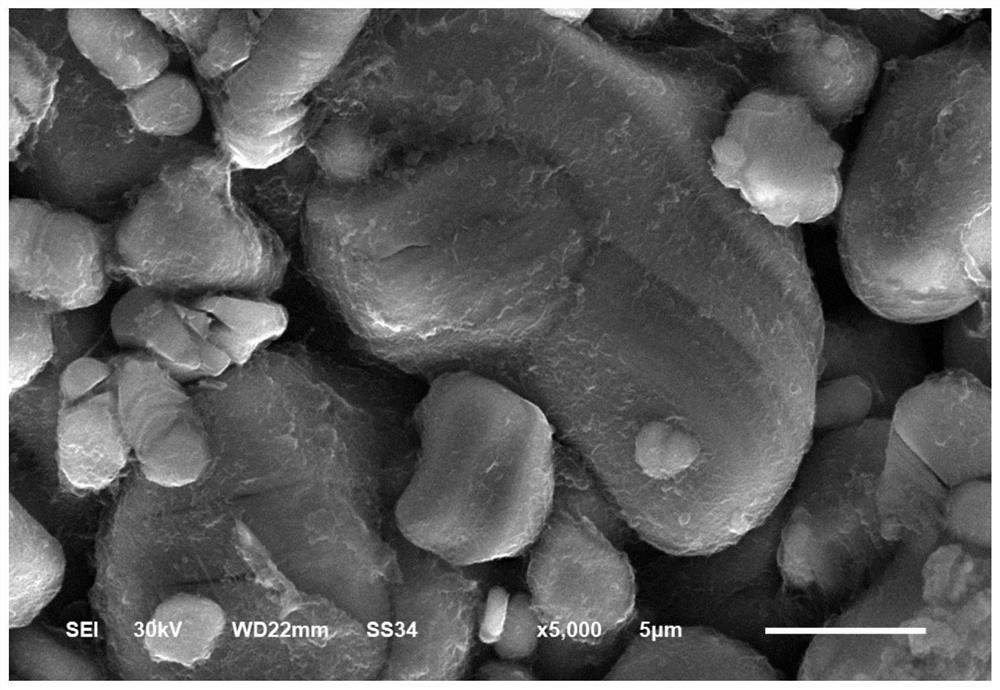
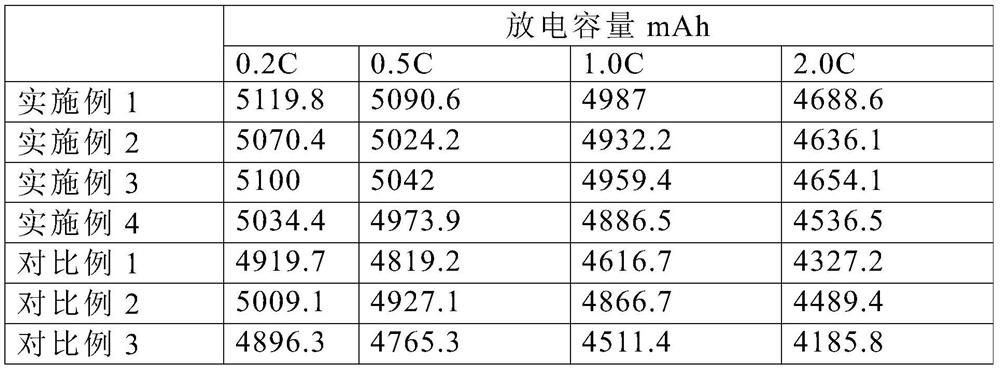
Image
Examples
preparation example Construction
[0026] In some embodiments of the present invention, the preparation method of the metaphosphate-coated lithium cobalt oxide material comprises: dissolving hydroxide in a phosphoric acid solution, reacting to obtain a dihydrogen phosphate solution, and then adding cobalt salt to adjust the pH 9-13, after further reaction, wash the obtained precipitate to neutrality, and dry to obtain the precursor;
[0027] The precursor, the lithium source, and the ethyl cellulose are dissolved in an organic solvent for ball milling, and the obtained sol-like mixture is dried and calcined to obtain a metaphosphate-coated lithium cobaltate material.
[0028] Control the reaction conditions so that hydroxide and phosphoric acid react to form dihydrogen phosphate, and dihydrogen phosphate and cobalt salt react under alkaline conditions to obtain a precursor, which is dissolved in an organic solvent with lithium source and ethyl cellulose After ball milling, drying, and calcination, the dihydroge...
Embodiment 1
[0039] Dissolve 0.2mol aluminum hydroxide in 0.6L 1mol / L phosphoric acid solution, stir and react at 140°C to obtain aluminum dihydrogen phosphate solution, then add 4mol cobalt sulfate, then drop in 4mol / L NaOH solution and 0.5mol / L Adjust the pH of the solution to 11 with ammonia solution, react at 60°C for 30 minutes, wash the obtained precipitate to neutrality, and dry it at 75°C to obtain the precursor;
[0040] Mix the precursor with 4.24 mol of lithium nitrate, and then add ethyl cellulose, which accounts for 1 wt% of the total amount of the precursor, lithium nitrate, and ethyl cellulose, and dissolve the mixture in ethanol and ball mill for 8 hours to form a sol The mixture was then dried at 150°C for 8 hours, and then heated to 850°C for 2 hours at a rate of 3°C / min and calcined for 2 hours. After sintering, it was cooled to obtain an aluminum metaphosphate-coated lithium cobaltate material.
Embodiment 2
[0042] Dissolve 0.3mol lithium hydroxide in 0.5mol / L phosphoric acid solution, adjust the amount of phosphoric acid solution added, so that the pH of the reaction end point is 2.5, and obtain a lithium dihydrogen phosphate solution, then add 10mol cobalt nitrate, and then drop in 3mol / L NaOH solution and 0.5 mol / L ammonia solution to adjust the pH of the solution to 10, further react at 50°C for 35 minutes, wash the obtained precipitate to neutrality, and dry at 80°C to obtain the precursor;
[0043] Mix the precursor with 5mol of lithium carbonate, then add ethyl cellulose, ethyl cellulose accounts for 1.5wt% of the total amount of the precursor, lithium carbonate, and ethyl cellulose, the mixture is dissolved in ethanol and ball milled for 7 hours to form a sol The mixture was then dried at 140°C for 8 hours, then heated to 750°C at a rate of 2°C / min and calcined for 2.5 hours, and cooled after sintering to obtain a lithium metaphosphate-coated lithium cobaltate material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com