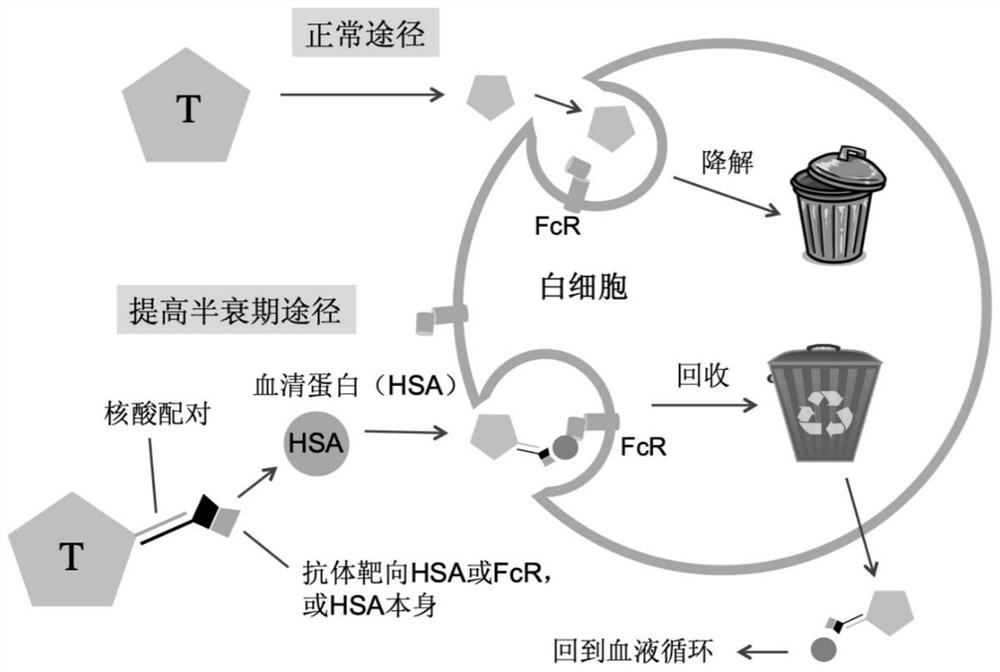
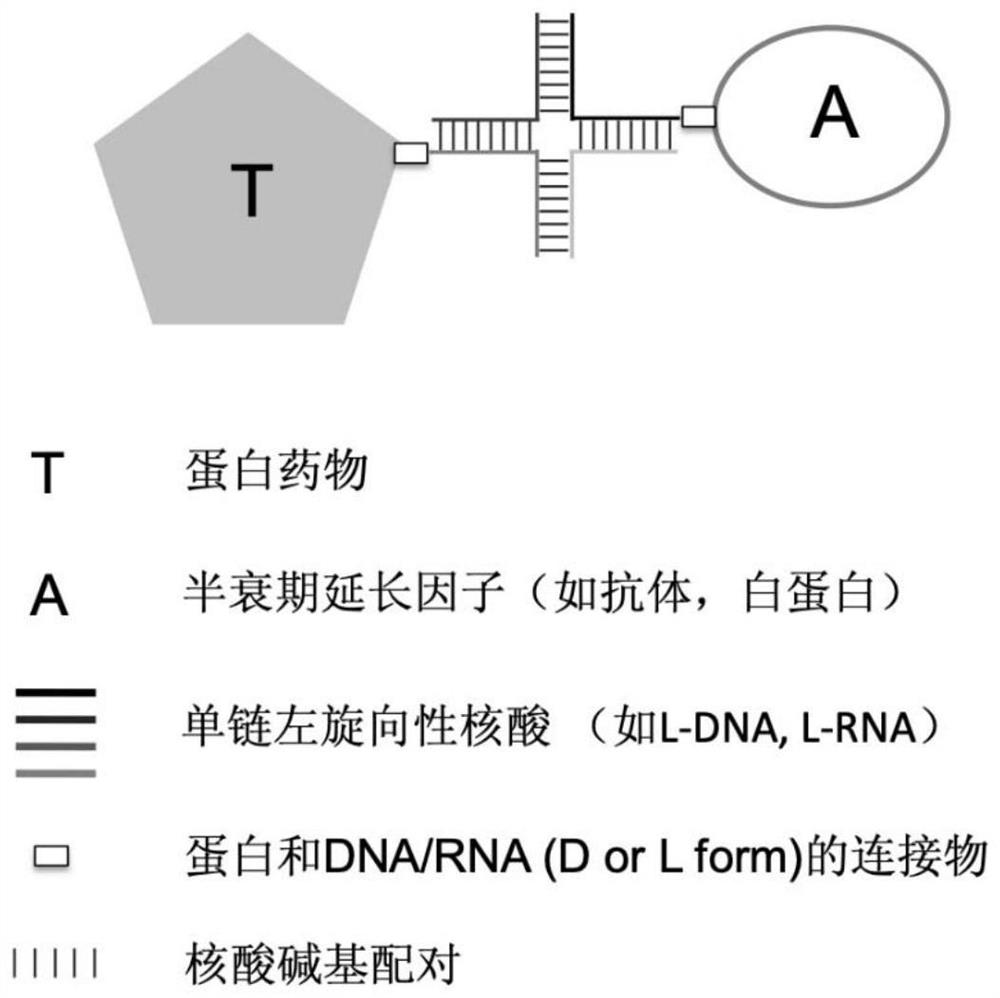
Drug with prolonged half-life period, library thereof, preparation method and application thereof
A half-life and drug technology, applied in the field of drugs with prolonged half-life and their libraries, can solve the problems of poor PEG molecular homogeneity, difficult quality control of PEGylated drugs, and limited half-life improvement effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0203] 2. Preparation method of antibody-L-nucleic acid complex
[0204] First, the 5' or 3' end of the L-nucleic acid is replaced by NH 2 Modification, and then according to the difference of the linker, the following main preparation methods can be used, wherein the functional group at one end of the linker is NHS (N-hydroxysuccinimide) or Sulfo-NHS (N-hydroxysuccinimide sulfonic acid sodium salt ), for rapid coupling of NH at one end of the L-nucleic acid 2group. Linkers containing bispecific functional groups are first combined with the NH of the L-nucleic acid 2 Reaction, followed by reducing the sulfhydryl group on the antibody, the group at the other end reacts with the sulfhydryl group to form a stable chemical bond.
[0205] 2.1 Maleimide. The linker used to conjugate the sulfhydryl group on the antibody is maleimide. Maleimides can rapidly react with free sulfhydryl groups on antibodies to form thioether bonds. Common linkers include SMCC (4-(N-maleimidomethyl)...
Embodiment 1 4
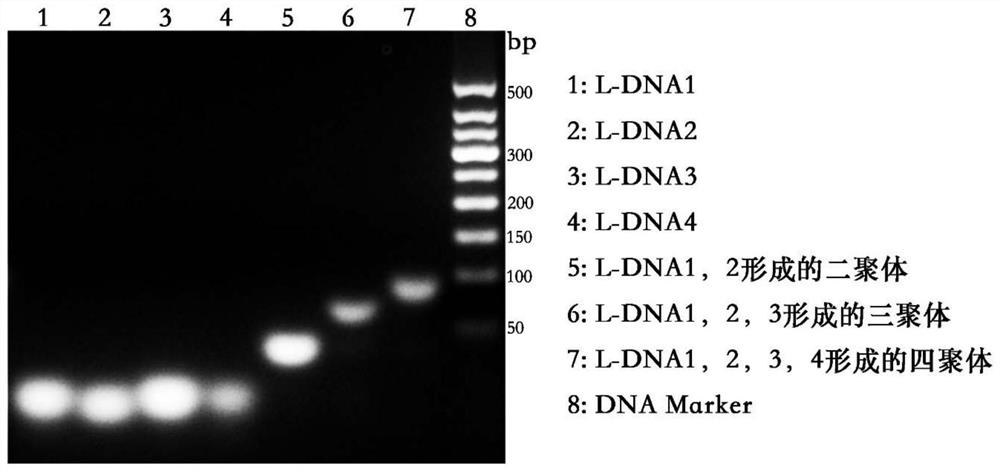
[0221] The design of embodiment 1 tetramer DNA framework
[0222] Design four L-nucleic acids that can be paired according to the quadrilateral shape (such as figure 2 shown). Wherein, any one L-nucleic acid single strand can perform specific complementary pairing with the other two L-nucleic acid single strands, but not pair with the fourth one. And the absolute value of the Gibbs energy change ΔG of the specific complementary pairing is much greater than that of the non-specific pairing, and the absolute value of the Gibbs energy change ΔG of the specific complementary pairing of each arm is greater than 25 kilocalories per mole (kcal / mole), while the non-specific pairings are all less than 7 kcal / mole (kcal / mole), which means that the tetramer assembly is easier to occur than the non-specific pairwise pairing, and the tetramer form is in the reaction system most stable.
[0223] The four L-DNA single-strand sequences designed according to the above principles are as fo...
Embodiment 2 4
[0233] Synthesis and Verification of Example 2 Tetramer DNA Framework
[0234] Synthesize 5'-terminal NH by biotechnology service companies (such as Chemgene Inc., Biosyn Inc., etc.) 2 Modified L-DNA single strands, the sequences of the four single strands are as shown in Example 1.
[0235] Phosphate buffer (50mM NaH 2 PO 4 , 150mM NaCl, pH 7.0) to dissolve the L-DNA single strand, and prepare a stock solution with a final concentration of 200uM. Dissolve SMCC powder with dimethyl sulfoxide (DMSO), and freshly prepare 250 mM SMCC stock solution. Add 10-50 times molar amount of SMCC mother solution to the L-DNA single-strand mother solution, mix rapidly and react at room temperature for 30min-2h. After the reaction was completed, 10% volume of 1M Tris-HCl (pH 7.0) was added to the reaction solution, mixed and incubated at room temperature for 20 minutes to terminate excess SMCC and continue the reaction. After the incubation, add 2 times the volume of 100% absolute ethano...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com