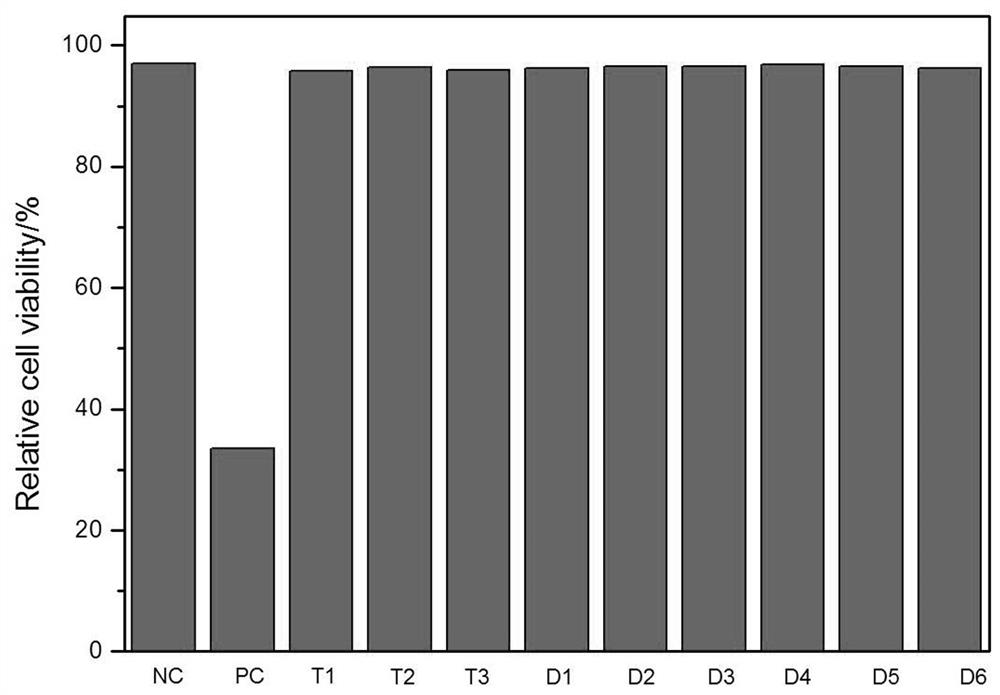
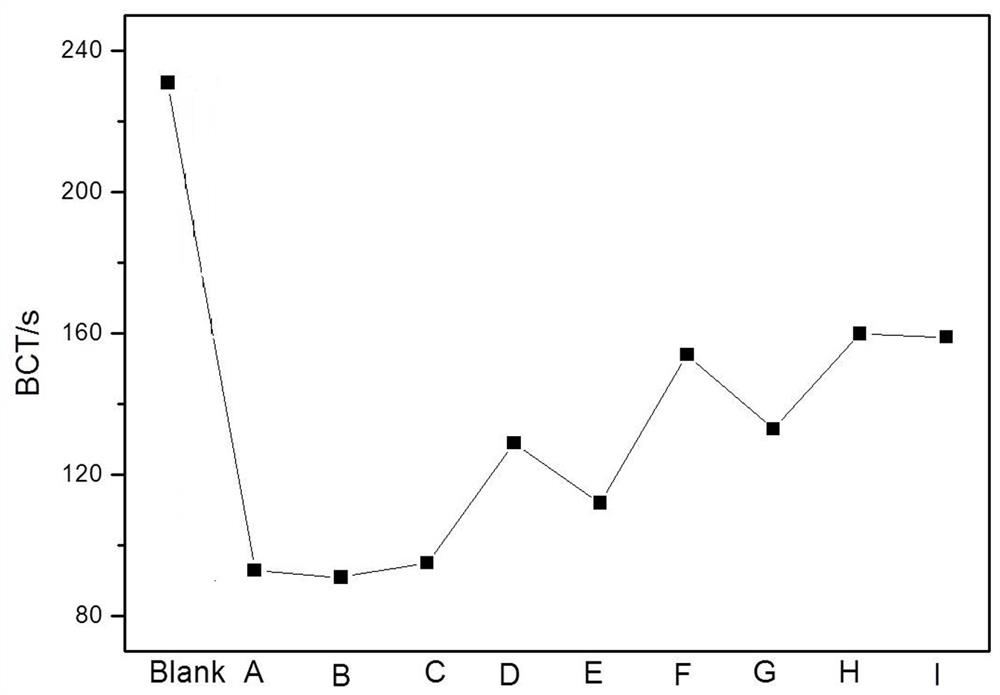
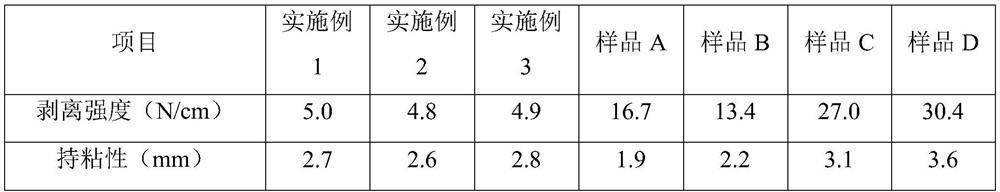
Antibacterial chitosan-based hemostatic patch
An antibacterial shell and hemostatic patch technology, applied in the field of biomedical materials, can solve the problems of secondary injury material comfort, powder instability, easy aggregation and hemostatic effect, etc., to promote adhesion and seal wounds, high activity and sterilization efficiency , Excellent anti-infection effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] An antibacterial chitosan-based hemostatic patch, comprising a backing layer, a hemostatic layer and a protective layer arranged in sequence from bottom to top; the backing layer is a base material prepared from polypropylene, coated with urethane ethyl acrylate dextran adhesive; the hemostatic layer includes a polylactic acid fiber non-woven fabric on the lower side and a composite hemostatic material on the upper side, and the ratio of parts by weight of the raw materials for preparing the composite hemostatic material is: modified 60 parts of chitosan, 2 parts of polyethylene glycol 2000, 16 parts of gelatin, 8 parts of xanthan gum, 5 parts of tea polysaccharide-nano-selenium complex, 4 parts of dopamine-modified nano-silicon dioxide, and 0.05 part of oleic acid amide; The protective layer is a PET film.
[0036] Further, the preparation method of the modified chitosan is: dissolving the chitosan powder in 1wt% acetic acid to form a 1wt% chitosan-acetic acid solution...
Embodiment 2
[0045] An antibacterial chitosan-based hemostatic patch, comprising a backing layer, a hemostatic layer and a protective layer arranged sequentially from bottom to top; the backing layer is a base material prepared from polyethylene terephthalate, coated with There is urethane methacrylate dextran adhesive; the hemostatic layer includes a polycarbonate non-woven fabric on the lower side and a composite hemostatic material on the upper side, and the parts by weight of the raw materials for preparing the composite hemostatic material are The ratio is: 90 parts of modified chitosan, 3 parts of polyethylene glycol 2000, 24 parts of gelatin, 12 parts of xanthan gum, 8 parts of tea polysaccharide-nano-selenium complex, 7 parts of dopamine-modified nano-silicon dioxide, oil 0.08 part of acid amide; the protective layer is a PET film.
[0046] Further, the preparation method of the modified chitosan is: dissolving the chitosan powder in 1wt% acetic acid to form a 1wt% chitosan-acetic ...
Embodiment 3
[0055] An antibacterial chitosan-based hemostatic patch, comprising a backing layer, a hemostatic layer and a protective layer arranged sequentially from bottom to top; the backing layer is a base material prepared from polyethylene terephthalate, coated with There is a urethane methacrylate dextran adhesive; the hemostatic layer includes a polyglycolic acid fiber non-woven fabric on the lower side and a composite hemostatic material on the upper side, and the weight part of the raw material for the composite hemostatic material is The ratio is: 120 parts of modified chitosan, 4 parts of polyethylene glycol 2000, 32 parts of gelatin, 16 parts of xanthan gum, 11 parts of tea polysaccharide-nano-selenium complex, 13 parts of dopamine-modified nano-silicon dioxide, 0.1 part of oleic acid amide; the protective layer is a PET film.
[0056] Further, the preparation method of the modified chitosan is: dissolving the chitosan powder in 1wt% acetic acid to form a 1wt% chitosan-acetic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com