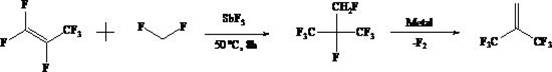Method for continuously preparing 3,3,3-trifluoro-2-(trifluoromethyl)-1-propene in gas phase
A technology of trifluoromethyl and fluoromethyl is applied in the field of gas-phase continuous preparation of 3,3,3-trifluoro-2--1-propene, which can solve the problem of low yield, low product yield and easy clogging of pipelines and other problems, to achieve the effect of long service life and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
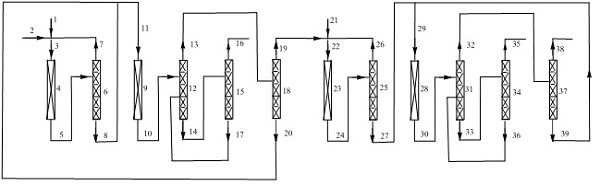
Image
Examples
preparation example Construction
[0061] 1. Preparation of hydrogenation catalyst:
[0062] (1) Preparation of the carrier of the hydrogenation catalyst: Dissolve aluminum trichloride in water, add concentrated ammonia water dropwise for precipitation, adjust the pH value to 7.5, then age for 24 hours, wash with water, filter, and dry in an oven at 120°C for 15 hour, the gained solid is pulverized, pressed into tablets, and the precursor of the carrier is obtained, and the precursor 10mL of the carrier is packed into a tubular reactor with an internal diameter of 1 / 2 inch and a length of 30cm. ℃ roasting for 12 hours, nitrogen space velocity is 200h -1 , and then lower the temperature to 300°C, and at the same time pass a mixed gas composed of hydrogen fluoride and nitrogen with a mass ratio of 1:2, and the total space velocity of the gas is 220h -1 , activated for 12 hours, stop the above gas mixture, and produce aluminum fluoride.
[0063] Other supports can be prepared by substituting other soluble metal ...
Embodiment 1
[0070] 2%Pd / 98%AlF is filled in a tubular reactor made of Incon alloy with an inner diameter of 1 / 2 inch and a length of 30cm 3 Catalyst 10mL. The reaction conditions are as follows: the reaction temperature is 50°C, the mass ratio of octafluoroisobutene to hydrogen is 1:10, the contact time is 60s, and the reaction pressure is 0.1MPa. After running for 10 hours, the reaction product was washed with water, washed with alkali and dried, and the gas phase organic phase was taken for GC analysis. The reaction results are: the conversion rate of octafluoroisobutene is 73.4%, and the selectivity of 1,1,1,3,3,3-hexafluoro-2-(difluoromethyl)propane is 99.8%.
Embodiment 2
[0072] 2%Pd / 98%AlF is filled in a tubular reactor made of Incon alloy with an inner diameter of 1 / 2 inch and a length of 30cm 3 Catalyst 10mL. The reaction conditions are as follows: the reaction temperature is 100°C, the mass ratio of octafluoroisobutene to hydrogen is 1:10, the contact time is 60s, and the reaction pressure is 0.1MPa. After running for 10 hours, the reaction product was washed with water, washed with alkali and dried, and the gas phase organic phase was taken for GC analysis. The reaction results are: the conversion rate of octafluoroisobutene is 93.8%, and the selectivity of 1,1,1,3,3,3-hexafluoro-2-(difluoromethyl)propane is 99.6%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com