Electrocobalt production method capable of recycling by-products
A production method and technology of by-products, applied in the field of electric cobalt production, can solve problems such as dangerous sources, prone to combustion and explosion, sodium chlorate production and transportation restrictions, and achieve the effect of solving supply problems and solving open circuit problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
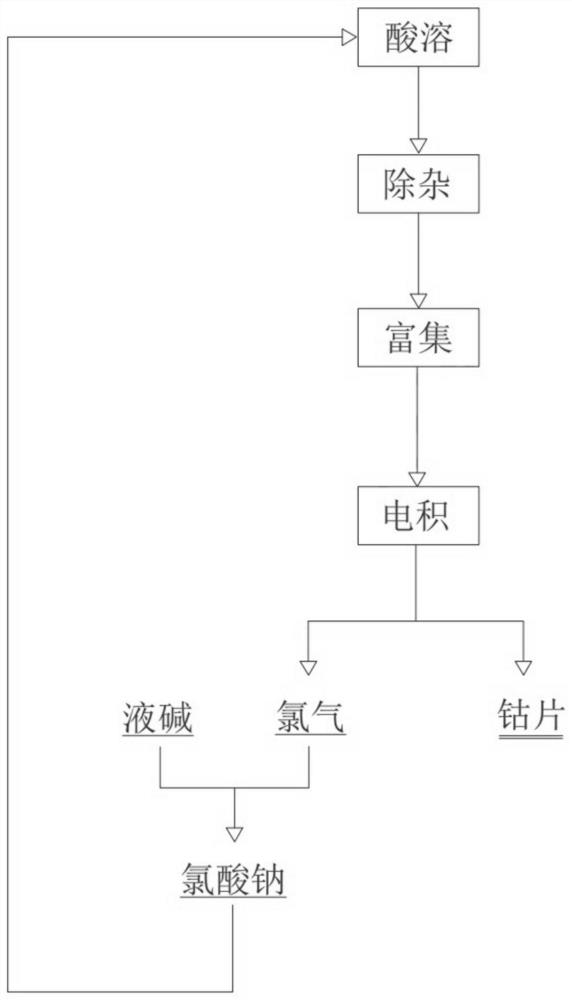
[0014] A method for producing electric cobalt with recyclable by-products, comprising the following steps: acid-dissolving an intermediate product of cobalt hydroxide, wherein the mass fraction of metal elements in the intermediate product of cobalt hydroxide is 30% for cobalt and 0.3% for copper. %, the manganese content is 1.8%, the nickel content is 0.06%, the iron content is 0.007%, the calcium content is 0.3%, the zinc content is 0.15%, and other impurities are acid-dissolved with hydrochloric acid, and the pH value is 1.0, and then removed Impurities and enrichment, specifically using chemical precipitation to remove iron, P204 to remove calcium, copper, zinc and manganese, 507 to remove nickel, and then continuous electrowinning, the reaction tank voltage is 2.8V, the current is 1A, and the temperature is 50°C. Cobalt flakes and chlorine gas are obtained after electrowinning , mix chlorine gas with liquid caustic soda, control the temperature of liquid caustic soda at 70...
Embodiment 2
[0017] A method for producing electric cobalt with recyclable by-products, comprising the following steps: acid-dissolving an intermediate cobalt hydroxide, wherein the mass fraction of metal elements in the intermediate cobalt hydroxide is 40% cobalt and 1.7% copper. %, the manganese content is 4%, the nickel content is 0.5%, the iron content is 0.09%, the calcium content is 0.7%, the zinc content is 0.25%, and other impurities are acid-dissolved with sulfuric acid, and the pH value is 1.5, and then removed Miscellaneous, enrichment, and then adopt continuous electrowinning, the reaction tank voltage is 3.5V, the current is 9000A, and the temperature is 70°C. After electrowinning, cobalt flakes and chlorine gas are obtained. The chlorine gas is mixed with liquid caustic soda, and the temperature of the liquid caustic soda is controlled at 75°C. The resulting mixed solution of sodium chlorate and sodium chloride returns to the acid solution together to achieve coarse impurity r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com