Hollow spherical bimetallic chalcogenide, preparation method and sodium battery negative electrode
A chalcogenide, hollow spherical technology, applied in battery electrodes, active material electrodes, negative electrodes, etc., can solve the problems of capacity loss, electrode material pulverization capacity decay, slow kinetics, etc., and achieves easy operation and low preparation cost. , easy-to-control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] A method for preparing a hollow spherical bimetallic chalcogenide material, comprising the following steps:
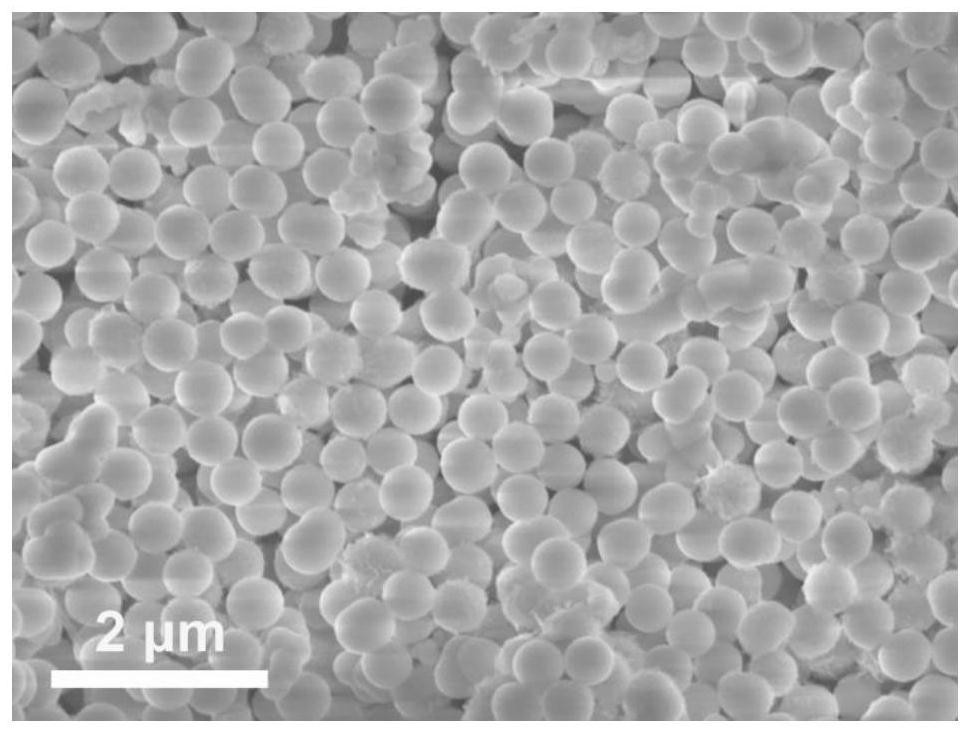
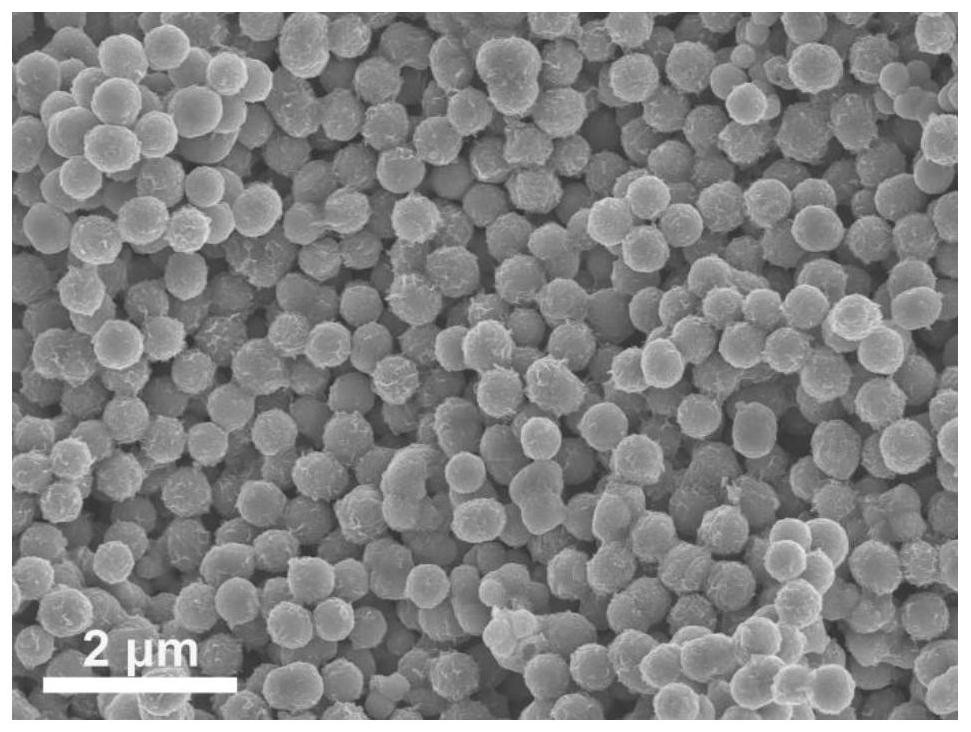
[0046] (1) 0.25mmol of Co(NO 3 ) 2 ·6H 2 O and 0.125mmol of Ni(NO 3 ) 2 ·6H 2 O was dissolved in 8 mL of glycerol and 40 mL of isopropanol to form a clear pink solution. The solution was then transferred to an autoclave and kept at 180 °C for 6 h. After naturally cooling to room temperature, the brown precipitate was separated by centrifugation, washed several times with deionized water and ethanol, and dried in an oven at 80 °C. Finally, the NiCo-glycerate precursor is obtained, and its SEM picture is attached figure 1 shown.
[0047] (2) Dissolve 30 mg of NiCo-glycerate precursor prepared in step (1) in 30 mL of deionized water, stir vigorously for about 30 min, and then dissolve 160 mg of Na 2 S·9H 2 O was added and stirred for 15-20 min to obtain a black solution. The solution was then transferred to an autoclave and kept at 160 °C for 8 h. After...
Embodiment 2
[0052] Graded spherical NiCo 2 S 4 The preparation of nanomaterials comprises the following steps:
[0053] (1) 0.25mmol of Co(NO 3 ) 2 ·6H 2 O and 0.125mmol of Ni(NO 3 ) 2 ·6H 2 O was dissolved in 8 mL of glycerol and 40 mL of isopropanol to form a clear pink solution. The solution was then transferred to an autoclave and kept at 180 °C for 6 h. After naturally cooling to room temperature, the brown precipitate was separated by centrifugation, washed several times with deionized water and ethanol, and dried in an oven at 80 °C. Finally, the NiCo-glycerate precursor is obtained, and its SEM picture is attached figure 1 shown.
[0054] (2) Dissolve 30 mg of NiCo-glycerate precursor prepared in step (1) in 30 mL of deionized water, stir vigorously for about 30 min, and then dissolve 160 mg of Na 2 S·9H 2 O was added and stirred for 15-20 min to obtain a black solution. The solution was then transferred to an autoclave and kept at 120 °C for 8 h. After natural cooli...
Embodiment 3
[0056] Graded spherical NiCo 2 S 4 The preparation of nanomaterials comprises the following steps:
[0057] (1) 0.25mmol of Co(NO 3 ) 2 ·6H 2 O and 0.125mmol of Ni(NO 3 ) 2 ·6H 2 O was dissolved in 8 mL of glycerol and 40 mL of isopropanol to form a clear pink solution. The solution was then transferred to an autoclave and kept at 180 °C for 6 h. After naturally cooling to room temperature, the brown precipitate was separated by centrifugation, washed several times with deionized water and ethanol, and dried in an oven at 80 °C. Finally, the NiCo-glycerate precursor is obtained, and its SEM picture is attached figure 1 shown.
[0058] (2) Dissolve 30 mg of NiCo-glycerate precursor prepared in step (1) in 30 mL of deionized water, stir vigorously for about 30 min, and then dissolve 160 mg of Na 2 S·9H 2 O was added and stirred for 15-20 min to obtain a black solution. The solution was then transferred to an autoclave and kept at 180 °C for 8 h. After natural cooli...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com