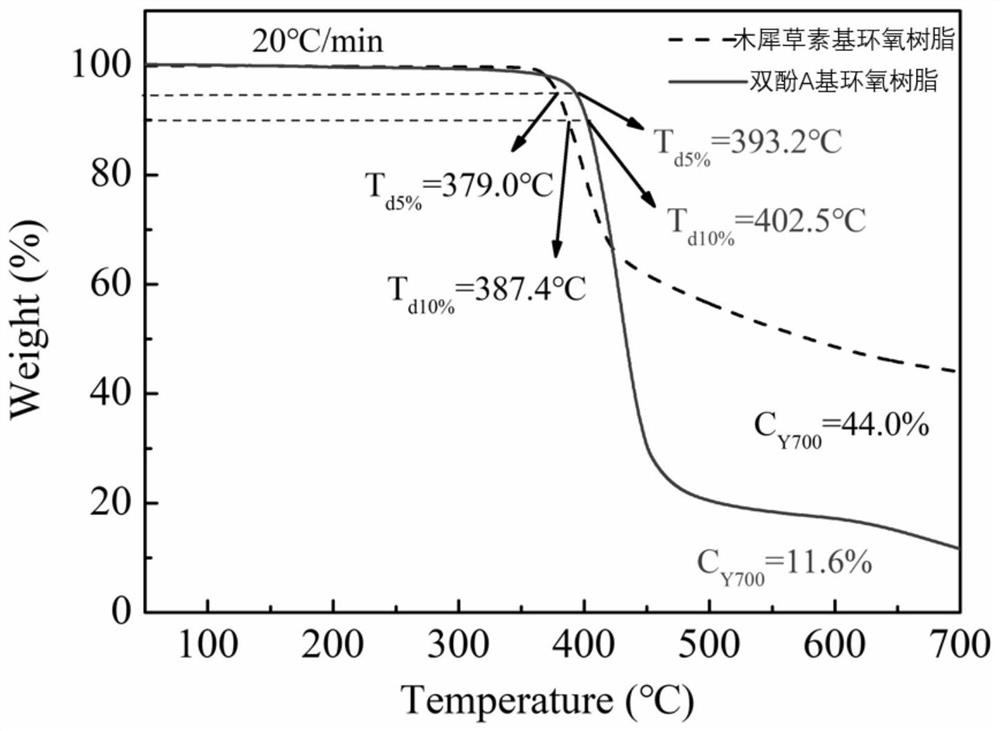
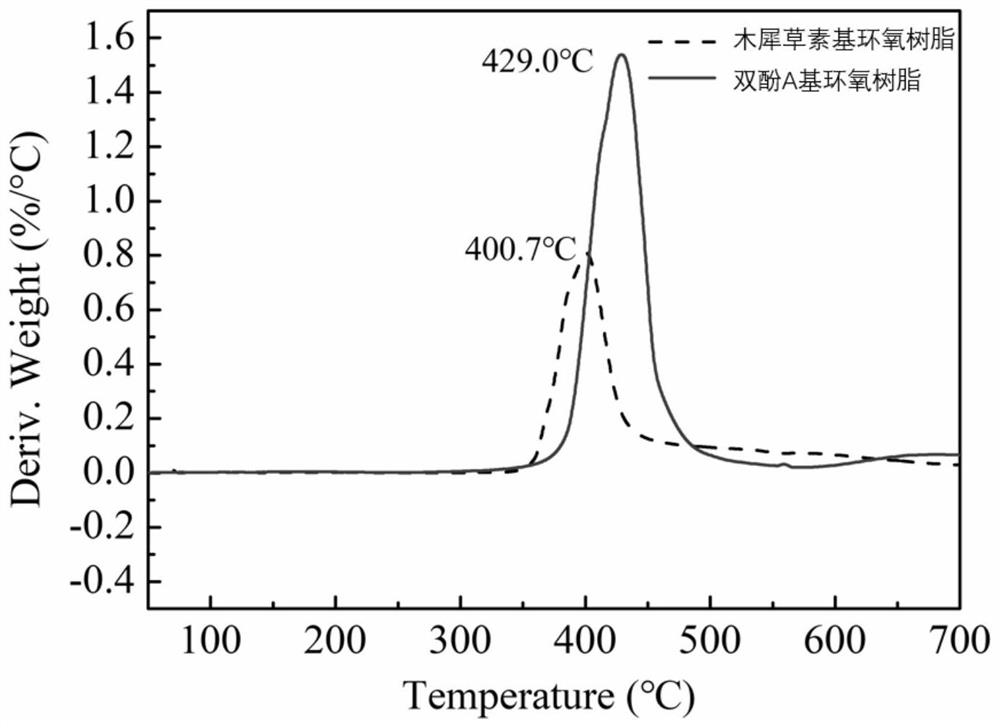
Luteolin-based epoxy resin and preparation method thereof
A technology based on epoxy resin and luteolin, which is applied in the direction of organic chemistry, can solve the problems that thermal and mechanical properties have not been significantly improved, unfavorable industrial production, and limited use range, etc., and is suitable for large-scale industrial production. Overall performance improvement and ease of operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 0.05mol luteolin, 1mol epichlorohydrin, and 3wt% tetrabutylammonium bromide into a 500mL three-necked flask equipped with a reflux condenser. The reaction was stirred at 105° C. for 3 h, cooled to room temperature, 50 wt % sodium hydroxide aqueous solution (0.5 mol sodium hydroxide) was added, and the reaction was stirred at room temperature for 5 h. After the reaction is finished, extract with dichloromethane and deionized water, dry with anhydrous magnesium sulfate for 12h, concentrate, and vacuum-dry to obtain the product (yield: 83%), and the light yellow solid obtained by column chromatography is the luteolin of the present invention Primer epoxy monomer.
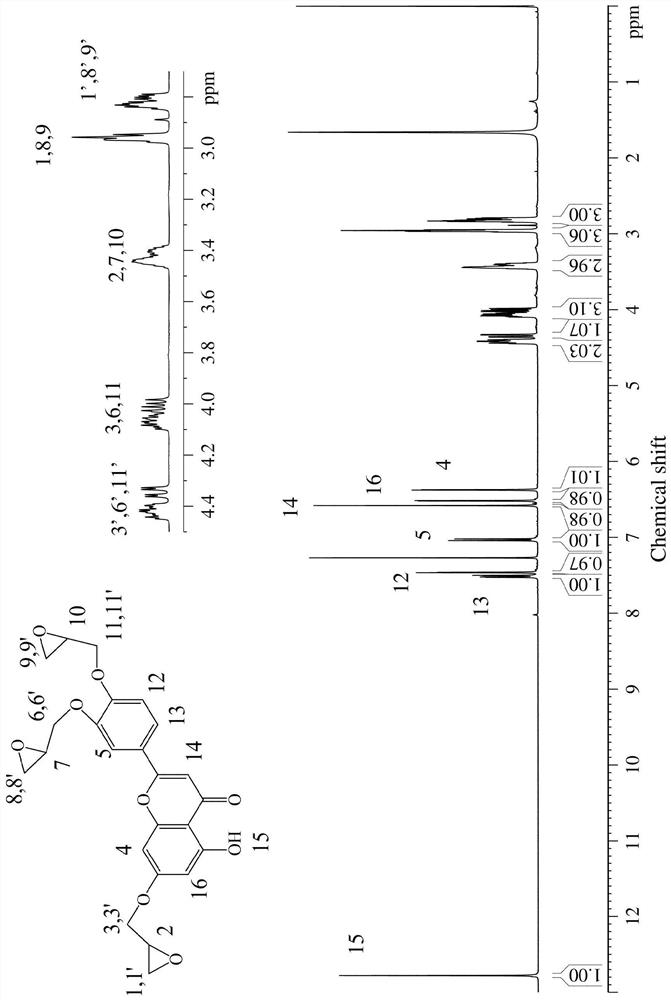
[0029] figure 1 For the luteolin-based epoxy monomer prepared in embodiment 1 1 H NMR spectrum, the specific analysis is as follows (δ, ppm): 2.79~2.85, 2.95~2.98, 3.38~3.47 (hydrogen on the epoxy group), 3.98~4.10, 4.33~4.36, 4.39~4.45 (connected epoxy -O-CH of group and benzene ring 2 ), 6.37~6.38, 6.5...
Embodiment 2
[0031] Add 0.05mol luteolin, 1mol epichlorohydrin, and 2wt% tetrabutylammonium bromide into a 500mL three-necked flask equipped with a reflux condenser. The reaction was stirred at 115° C. for 3 h, cooled to room temperature, 50 wt % sodium hydroxide aqueous solution (0.5 mol sodium hydroxide) was added, and the reaction was stirred at room temperature for 3 h. After the reaction is finished, extract with dichloromethane and deionized water, dry with anhydrous magnesium sulfate for 12h, concentrate, and vacuum-dry to obtain the product (yield: 82%), and the light yellow solid obtained by column chromatography is the luteolin of the present invention Primer epoxy monomer.
Embodiment 3
[0032] The synthesis of embodiment 3 luteolin-based epoxy monomers
[0033] Add 0.05mol luteolin, 2mol epichlorohydrin, and 4wt% benzyltriethylammonium chloride into a 500mL three-necked flask equipped with a reflux condenser. The reaction was stirred at 125° C. for 1 h, cooled to room temperature, 30 wt % potassium hydroxide aqueous solution (0.5 mol sodium hydroxide) was added, and the reaction was stirred at room temperature for 5 h. After the reaction is finished, extract with dichloromethane and deionized water, dry with anhydrous magnesium sulfate for 12h, concentrate, and vacuum-dry to obtain the product (yield: 87%), and the light yellow solid obtained by column chromatography is the luteolin of the present invention Primer epoxy monomer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com