Stilbene compound separated from rheum lhasaense and application of stilbene compound in treatment of nervous system diseases
A technology of stilbene and Lhasa rhubarb, which is applied to nervous system diseases, medical preparations containing active ingredients, drug combinations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Separation and Characterization of Stilbene Compounds from Rhubarb Lhasa
[0039] 1. Extraction and separation of stilbene compounds from Lhasa rhubarb
[0040] The Lhasa rhubarb medicinal material is crushed, extracted with 95% ethanol, and concentrated under reduced pressure to obtain the extract. After extraction, it is divided into chloroform part, ethyl acetate part, n-butanol part and water part. The samples from the ethyl acetate part were loaded on the MCI packed chromatography column, and were rinsed with 30%, 50%, 70% and 100% ethanol solvents respectively, and each solvent rinsed 4-5 column volumes. Among them, compounds 1 and 2 were isolated from the 50% ethanol fraction, and compound 3 was isolated from the 70% fraction.
[0041] 2. Structural analysis of new compounds
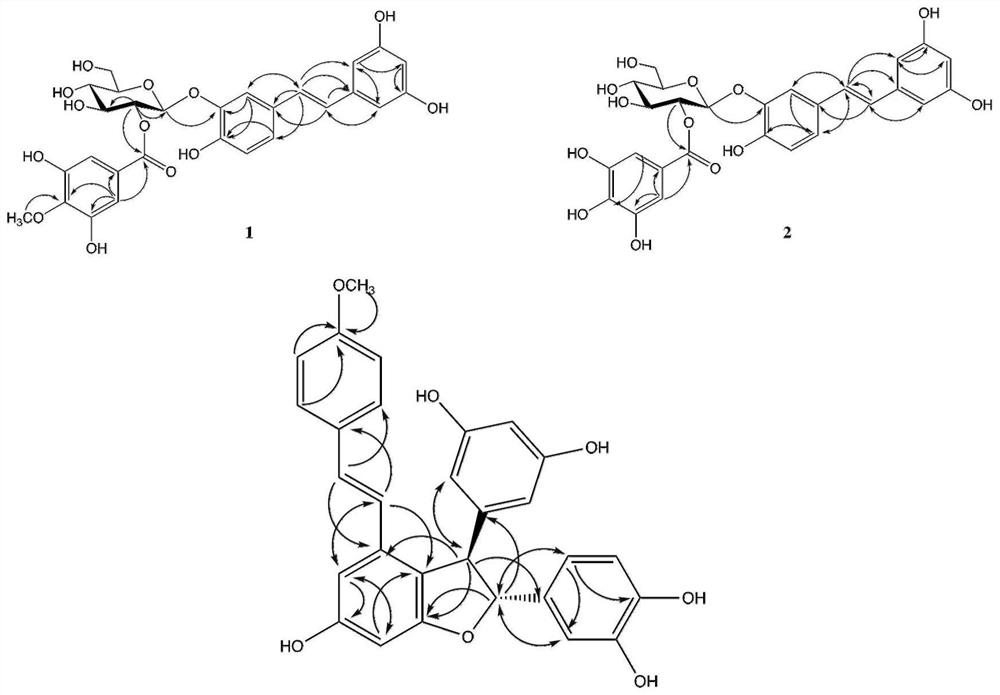
[0042] Compound 1: brown powder, molecular ion peak m / z 595.1423 [M+Na] given by (+)HRESIMS + (calcd for 595.1428, see attached figure 1 ) to determine its molecular formula...
experiment example 1
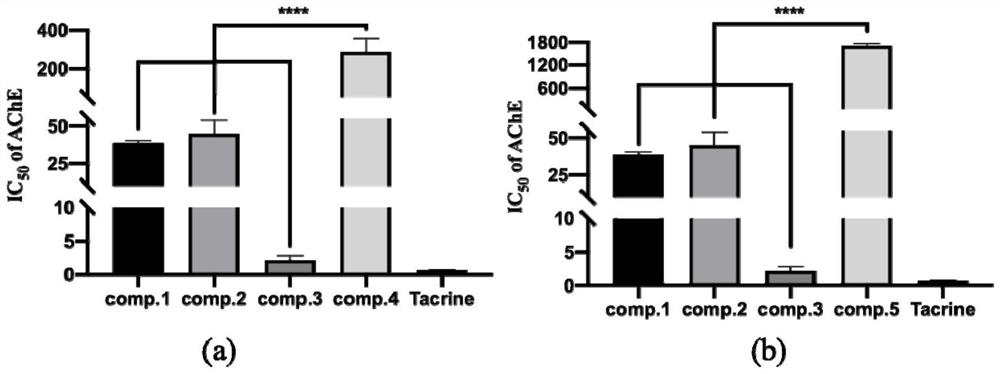
[0052] Experimental example 1 Acetylcholinesterase (AChE) inhibitory activity screening experiment
[0053] 1. Test method
[0054] Acetylcholinesterase inhibitory activity is based on the improved Ellman method, and the inhibitory effect of compounds 1 and 2 on AChE was measured in a 96-well plate. The reagents used in the experiment include the reaction substrate ATCI, the chromogenic agent DTNB and the reaction terminator in 1% SDS buffer containing (1g SDS in PBS (ph8.0)). The assay steps are as follows: first, mix 60 μL of PBS (pH 8.0), 10 μL of 3 mM DTNB, 20 μL of the test compound and 20 μL of 0.50 U / mL AChE solution in a 96-well plate, and incubate at 37°C for 10 minutes to activate the enzyme; secondly, the 10 μL of substrate (ATCI) was added to the microplate and incubated at 37°C for 15 minutes. Third, add 80 μL of 1% SDS solution to stop the reaction. Finally, the absorbance was measured at 405 nm using a multi-functional microplate reader (Tecan Infinite M200, ...
experiment example 2
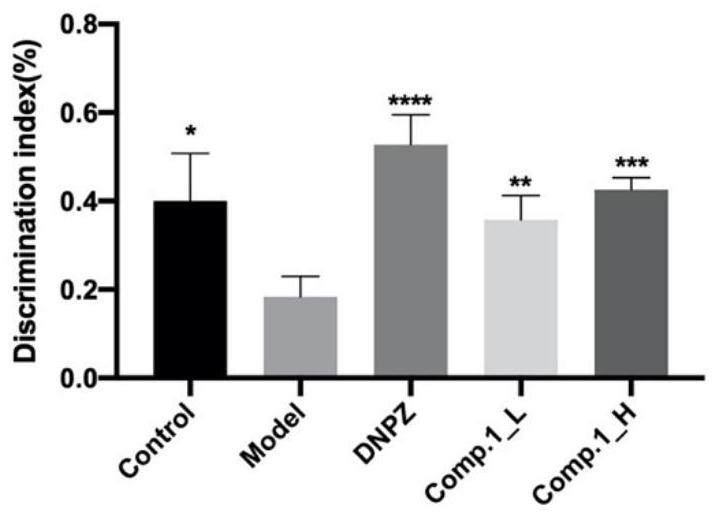
[0063] Experimental Example 2 Animal Experiment and Behavioral Experiment of Compound 1
[0064] 1 Experimental method
[0065] 1.1 Animal experiments
[0066] Animal experiments mainly study the new compound 1 (comp.1, picatanol-3'-O-β- D -[2″-(3,5-dihydroxy-4-methoxybenzoyl)]-glucopyranoside) preventive effect on cognitive impairment. 60 SPF grade C57 male mice of 8 weeks old ( Purchased from Speiford (Beijing) Biotechnology Co., Ltd.), fed in plastic cages, free access to food and water, room temperature 25 ± 2 ° C, humidity 55 ± 10%, maintaining a circadian rhythm of 12h light and 12h dark. Male mice were randomly divided into 5 groups (12 mice per group): (1) blank control group (Control, Vehicle), (2) model group (Model, Scopolamine, 1.5 mg·kg -1 ), (3) Positive drug group (DNP, Scopolamine+Donepezil, 3.0mg·kg -1 ), (4) low dose group (Compound 1, Scopolamine+comp.1, 70mg·kg -1 ), (5) high dose group (Compound 1, Scopolamine+comp.1, 350mg·kg -1 ). The mice were gi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com