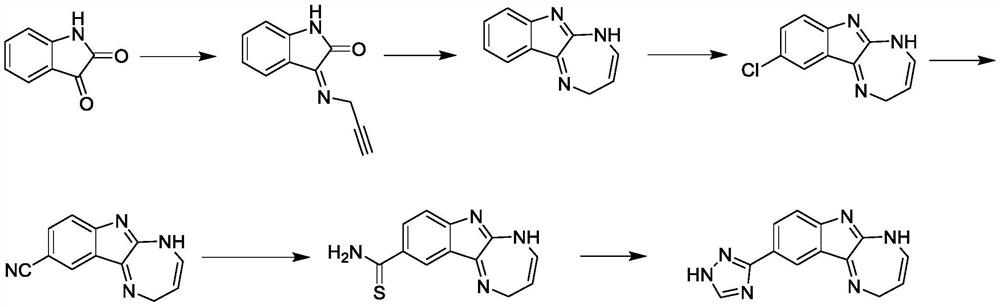
A kind of indole drug molecule for medical care, sterilization and disinfection, its preparation method and application
A drug molecule, sterilization and disinfection technology, applied in the fields of botanical equipment and methods, applications, disinfectants, etc., can solve the problem of infiltration diagnosis and treatment technology that cannot be guaranteed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
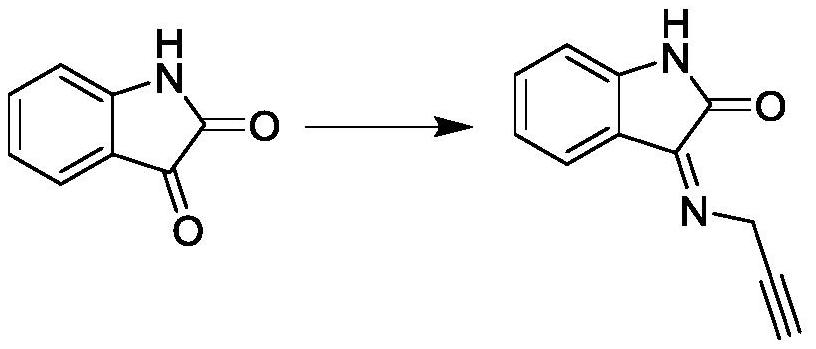
Embodiment 1
[0029]
[0030] In a reaction flask with argon protection, add 1.1g of 3-aminopropyne and 1.5g of isatin into 50mL of N,N-dimethylformamide, stir to dissolve completely, and then add cuprous bromide 0.15g, cesium carbonate 3.2g and o-phenanthroline 0.36g, heated to 90°C under argon protection, reacted for 0.5h, poured into 100mL of water, extracted several times with 20mL of dichloromethane, combined the organic phases, concentrated After purification by column chromatography, 1.71 g of the alkynyl compound was obtained; LC-MS (ESI): m / z 185 [M+H] + ;Elemental analysis calculated value [C 11 h 8 N 2 O]: C, 71.73; H, 4.38; N, 15.21. Found: C, 71.68; H, 4.36; N, 15.25.
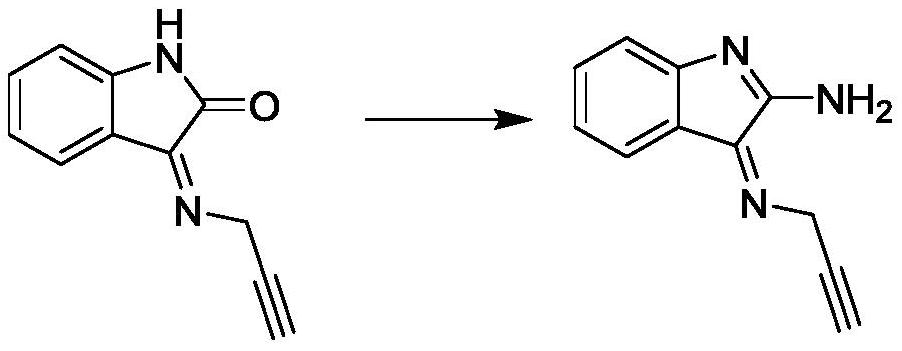
Embodiment 2
[0032]
[0033] Add 18.5g of alkynyl compound and 15.5g of phosphorus oxychloride to 400mL of chloroform in a reaction bottle with argon protection, heat to reflux for 2h, then wash the reaction liquid with water, separate the organic phase, concentrate and add 50mL of phenol and xylene 450mL, stir and dissolve, add potassium hydroxide 11g, heat to reflux, remove the moisture in the reaction system through a water separator, then add ammonium acetate 15g, continue to heat to reflux, react for 3h, slowly drop to room temperature, and then Pour the reaction solution into 500mL of water, extract it several times with 200mL of ethyl acetate, combine the organic phases, adjust the pH of the organic phase to neutral with dilute hydrochloric acid, then adjust the pH to 8-9 with saturated potassium hydroxide solution, and divide again. The organic phase was taken out, concentrated and separated by silica gel column chromatography to obtain 9.3 g of amine compounds; LC-MS (ESI): m / z ...
Embodiment 3
[0035]
[0036] In a reaction flask with nitrogen protection, add 18.5g of alkynyl compound and 24g of phosphorus oxychloride to 600mL of chloroform, heat to reflux for 3h, then wash the reaction solution with water, separate the organic phase, add 50mL of phenol and In 500mL of xylene, stir and dissolve, add 16g of potassium hydroxide, heat to reflux, remove the moisture in the reaction system through a water separator, then add 15g of ammonium acetate, continue to heat to reflux, react for 4h, slowly drop to room temperature, and then Pour the reaction liquid into 500mL of water, extract it several times with 200mL of ethyl acetate, combine the organic phase, adjust the pH of the organic phase to neutral with dilute hydrochloric acid, then adjust the pH to 8-9 with saturated potassium hydroxide solution, and separate The organic phase was concentrated and separated by silica gel column chromatography to obtain 17.69 g of amine compounds; LC-MS (ESI): m / z 184 [M+H] + ; 1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com