Phenolic acid polypeptide conjugate as well as preparation method and application thereof
A technology of polypeptide coupling and phenolic acid, which is applied in the field of medicine, can solve the problems of reduced physiological activity, limited application range, and low safety, and achieve the effects of improved safety, reduced by-product formation, and increased yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] In this example, gallic acid-polypeptide conjugates were prepared by the following method, specifically including the following steps:
[0051] (1) Polypeptide solid-phase synthesis method using FMOC strategy, using amino acid protected by FMOC at the amino terminal as raw material, using Liberty Lite automatic microwave peptide synthesizer from CEM Company to synthesize, and the operation method was carried out according to the instrument manual.
[0052] Synthetic reagent selection:
[0053] (a) Carrier resin: 2-chlorotrityl chloride resin, degree of substitution: 0.5.
[0054] (b) Deprotection reagent: 20% piperidine in DMF.
[0055] (c) Coupling reagent: HOBT, acid-binding agent: DIEA are used in the condensation reaction.
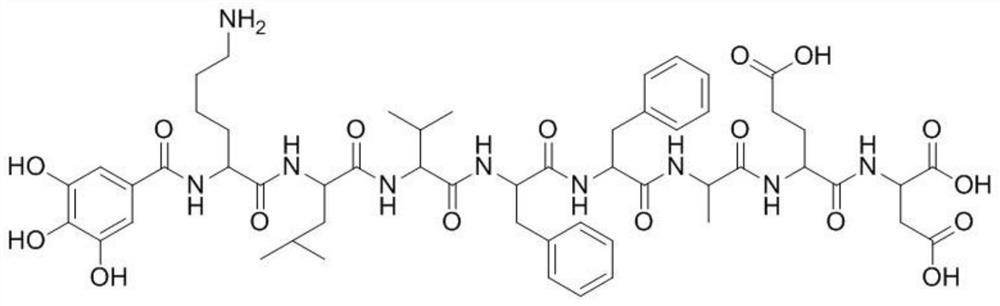
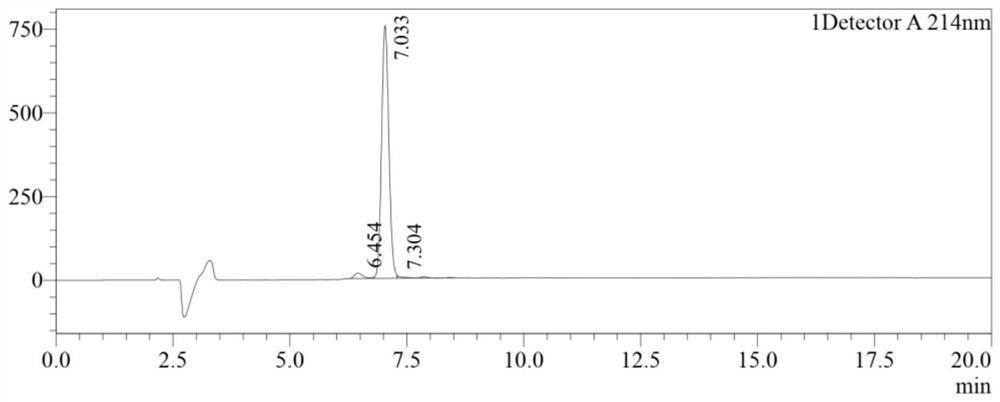
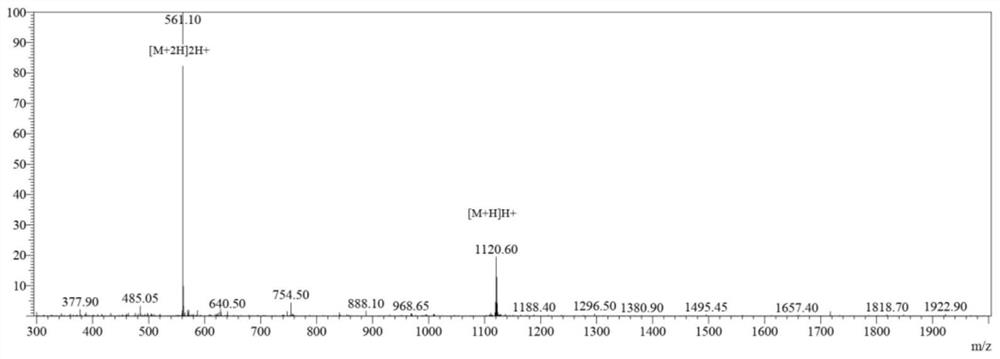
[0056] (2) Connect the required amino acids in the polypeptide sequence from right to left. After the reaction is completed and the KLVFFAED polypeptide is synthesized, gallic acid, PyBOP, and DIEA are dissolved in DMF and coupled with the res...
Embodiment 2
[0060] In this example, gallic acid-polypeptide conjugates were prepared by the following method, specifically including the following steps:
[0061] (1) Polypeptide solid-phase synthesis method using FMOC strategy, using amino acid protected by FMOC at the amino terminal as raw material, using Liberty Lite automatic microwave peptide synthesizer from CEM Company to synthesize, and the operation method was carried out according to the instrument manual.
[0062] Synthetic reagent selection:
[0063] (a) Carrier resin: 2-chlorotrityl chloride resin, degree of substitution: 0.5.
[0064] (b) Deprotection reagent: 20% piperidine in DMF.
[0065] (c) Coupling reagent: HOBT, acid-binding agent: DIEA are used in the condensation reaction.
[0066] (2) Connect the required amino acids in the polypeptide sequence from right to left. After the reaction is completed and the KLVFFAED polypeptide is synthesized, gallic acid, DMAP, and DIEA are dissolved in DMF and coupled with the resi...
Embodiment 3
[0070] In this example, gallic acid-polypeptide conjugates were prepared by the following method, specifically including the following steps:
[0071] (1) Polypeptide solid-phase synthesis method using FMOC strategy, using amino acid protected by FMOC at the amino terminal as raw material, using Liberty Lite automatic microwave peptide synthesizer from CEM Company to synthesize, and the operation method was carried out according to the instrument manual.
[0072] Synthetic reagent selection:
[0073] (a) Carrier resin: 2-chlorotrityl chloride resin, degree of substitution: 0.5.
[0074] (b) Deprotection reagent: 20% piperidine in DMF.
[0075] (c) Coupling reagent: HOBt, acid binding agent: DIEA are used during the condensation reaction.
[0076] (2) Connect the required amino acids in the polypeptide sequence from right to left. After the reaction is completed and the KLVFFAED polypeptide is synthesized, gallic acid, PyBOP, and TEA are dissolved in DMF and coupled with the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com