Semi-synthesis method of abietane diterpene and derivative thereof, abietane diterpene derivative and application
A technology of rosinane diterpene and synthesis method, applied to semi-synthesis of rosinane diterpenes and derivatives thereof, rosin alkane diterpenes derivatives and application fields, can solve the problem of excessive time consumption, low nepetaefolin and limited biological activity In-depth research and other issues to achieve strong anti-tumor activity and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
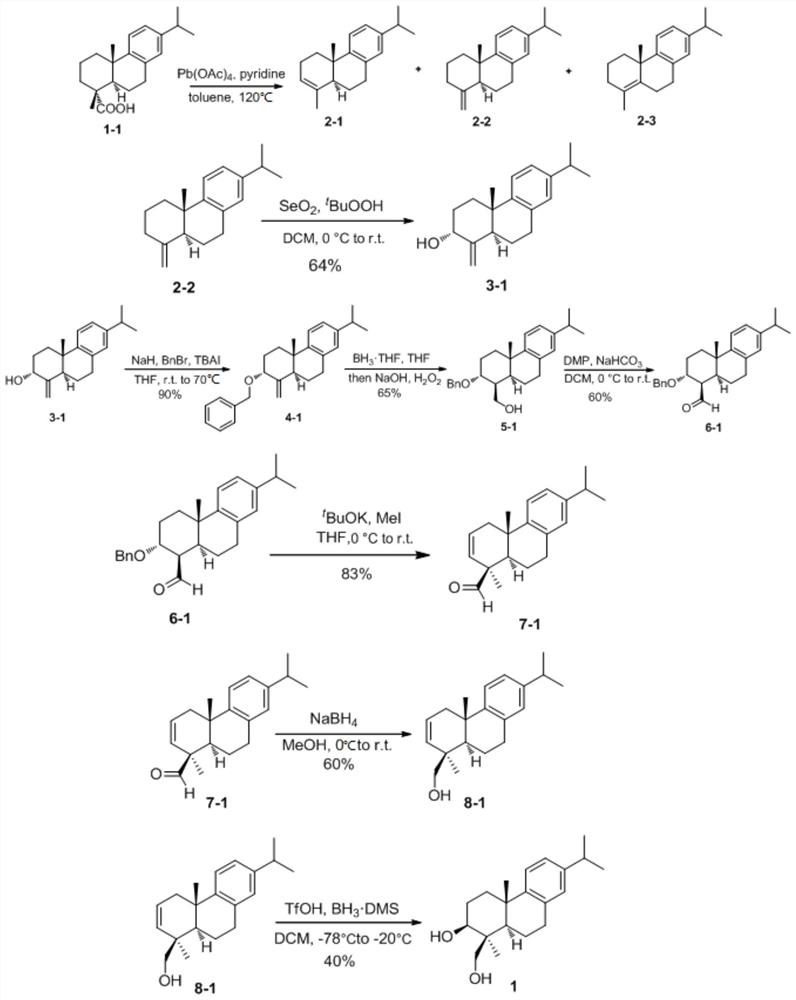
[0042] The structural formula of the abietane diterpene of present embodiment 1 is as follows:
[0043]
[0044] The semi-synthetic method of this abietane diterpene comprises the following steps:
[0045] 1) Pour the raw material dehydroabietic acid 1-1 (25g, 83mmol) into an eggplant-shaped bottle, add magneton and solvent toluene 125ml, then add 15ml of ultra-dry pyridine, lead tetraacetate (74g, 207.5mmol), After heating and refluxing in an oil bath at 120°C for 2 hours, the conversion of the raw materials was complete, and the reaction system was filtered through diatomaceous earth to obtain a brown clear solution, and the toluene was spin-dried by an oil pump at 40°C. Then perform silica gel column chromatography, PE can elute the product, and the three products cannot be separated, and the hydrogen nuclear magnetic spectrum shows that the ratio of the three products obtained by the reaction is about 3:3:2. After concentration, the colorless oil 2-1-2-3 (21.4 g) was o...
Embodiment 2
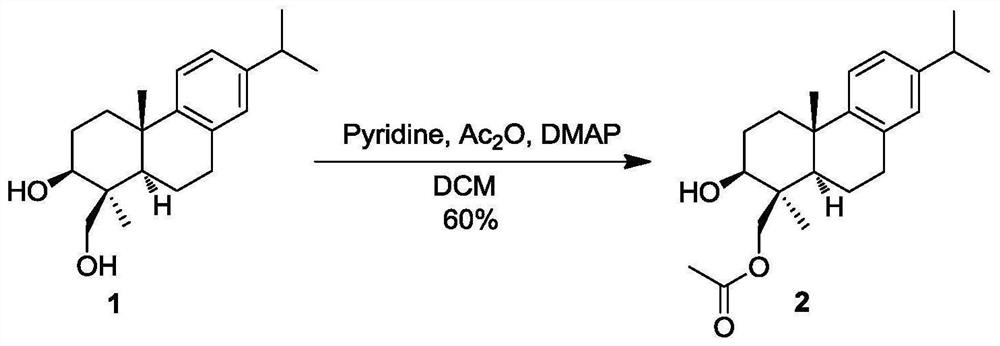
[0054] The structural formula of the abietane diterpene derivative of present embodiment 2 is as follows:
[0055]
[0056] Take a dry eggplant-shaped flask, add substrate 1 (40 mg, 0.133 mmol), and replace the argon gas 3 times. Under the protection of argon, the above compound was dissolved in anhydrous dichloromethane (2ml), and DMAP (1.7mg, 0.0133mmol) and pyridine (21.4μl, 0.266mmol) were added successively, and acetic anhydride (13.7μl, 0.146mmol) was injected after reacting for 2min. mmol), overnight reaction. The next day, TLC showed that the raw material was still remaining, and there was a by-product protected by the 3-hydroxyacetyl group. NaHCO 3 The reaction was quenched with aqueous solution, extracted with ethyl acetate (4×2mL), the combined organic phases were washed with water, dried over anhydrous sodium sulfate, concentrated by filtration, and purified by flash column chromatography (PE / EA=5:1) to obtain a white solid, which Structural formula such as 2...
Embodiment 3
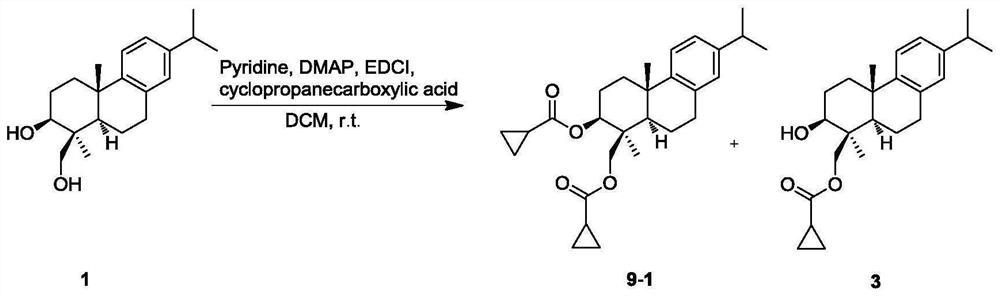
[0075] The structural formula of the abietane diterpene derivative of present embodiment 3 is as follows:
[0076]
[0077] Propionic acid was condensed with 20 mg of triptobenzene L (structural formula 1) to obtain the double-substituted product 9-1 and the mono-substituted product 19-cyclopropanecarboxyl-triptobenzene L (structural formula 3), respectively. After silica gel column chromatography, petroleum ether-ethyl acetate Gradient elution was carried out in the ester solvent system, and the derivatives of triptobenzene L were obtained by separation and purification, the structural formula of which was shown in 3.
[0078] The physical and chemical constants and hydrocarbon data of 19-cyclopropanecarboxyl-triptobenzene L:
[0079] white solid, 1 H NMR (400MHz, CDCl 3)δ7.16(d, J=8.2Hz, 1H), 7.00(dd, J=8.1, 2.1Hz, 1H), 6.89(d, J=2.0Hz, 1H), 4.45(d, J=11.7Hz, 1H), 4.23(d, J=11.7Hz, 1H), 3.35(dd, J=11.4, 4.9Hz, 1H), 2.94(dd, J=17.0, 6.1Hz, 1H), 2.83(overlapped, 2H), 2....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com