Alkylated hydrophobic MOFs material and application thereof in cyclohexene hydration
An alkylation and alkylation modification technology, applied in the field of chemical engineering, can solve problems such as low conversion rate, and achieve the effects of improving catalytic efficiency, good application prospects, and increasing contact area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 1. Preparation of alkylated hydrophobic MOFs material UiO-66-HAc-OTS:
[0042] (1) Weigh 0.932ZrCl respectively 4 , 1.32g H 2 Dissolve BDC (1,4 phthalic acid) and 5 mL acetic acid in 24 mL DMF (N, N-dimethylformamide), and sonicate for 30 min to obtain solution A;
[0043] (2) After solution A is completely dissolved, transfer it to a reaction kettle lined with polytetrafluoroethylene, seal it, and heat it statically at 120°C for 24 hours;
[0044] (3) When the crystallization is completed and cooled to room temperature, the solution A is centrifuged, and the solid is retained, washed three times with N,N-dimethylformamide, and then washed three times with methanol to remove residual reactants and exchange N , N-dimethylformamide, after washing, white powdery product is obtained;
[0045] (4) Transfer the white powdery sample obtained in step (3) to a watch glass, and put it in a vacuum drying oven, set 100 ° C, and vacuum dry for 24 hours to obtain UiO-66-Hac; (step...
Embodiment 2
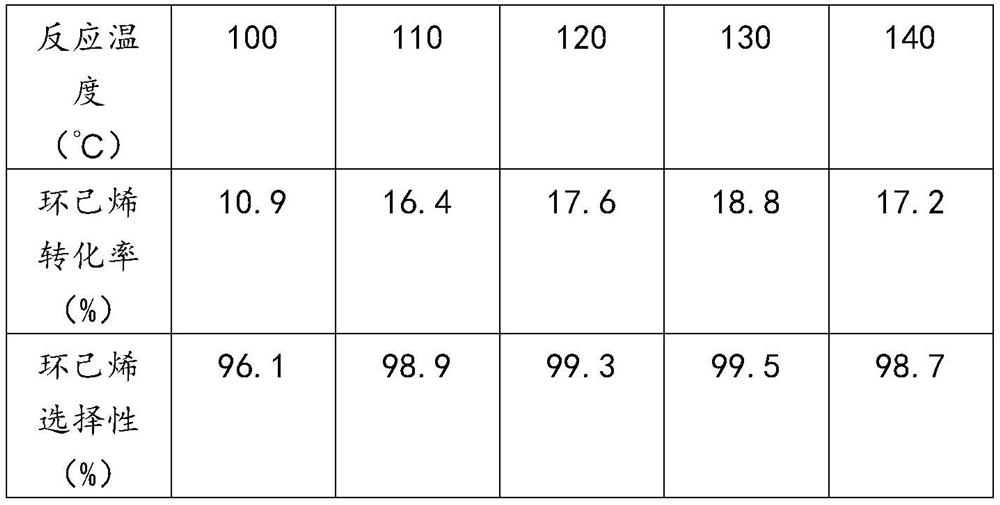
[0055] The UiO-66-HAc-OTS obtained in Example 1 was used to catalyze the hydration reaction of cyclohexene, and the reaction conditions were referred to step 2 of Example 1, except that the selected hydration reaction temperature was different. The selection of the reaction temperature and the reaction results are shown in Table 1.
[0056] The influence of table 1 reaction temperature on cyclohexene hydration reaction
[0057]
[0058] It can be seen from Table 1 that the hydration of cyclohexene is a reversible exothermic reaction. Increasing the reaction temperature effectively increases the effective collision between molecules and accelerates the reaction rate. At the same time, the adsorption rate of cyclohexene and the desorption rate of cyclohexanol on the catalyst surface are both accelerated. The reaction conversion rate is effectively improved. When the reaction temperature is too low, the molecular activity is low and the conversion rate is not high; when the r...
Embodiment 3
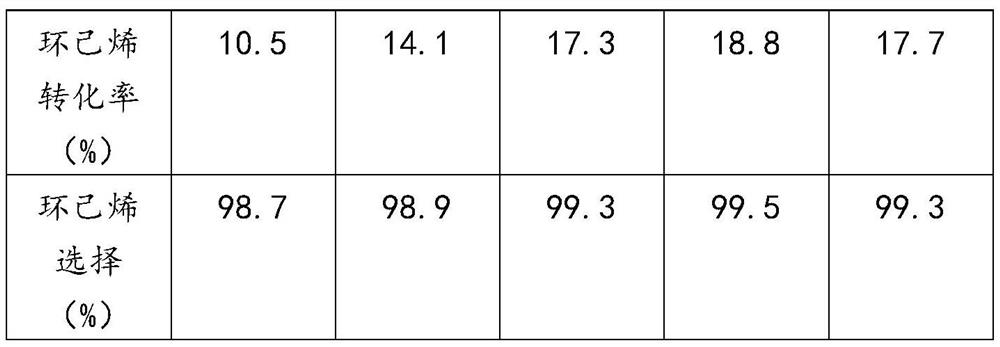
[0060] The UiO-66-HAc-OTS obtained in Example 1 was used to catalyze the hydration reaction of cyclohexene, and the reaction conditions were referred to step 2 of Example 1, except that the selected hydration reaction time was different. The selection of reaction time and reaction results are shown in Table 2.
[0061] The influence of table 2 reaction time on cyclohexene hydration reaction
[0062]
[0063]
[0064] As can be seen from Table 2, the reaction time has little influence on the selectivity of cyclohexanol, but has a certain influence on the conversion rate of cyclohexene. When the reaction time was 1h, the conversion rate of cyclohexene was 10.5%; when the reaction time was increased to 4h, the conversion rate of cyclohexene reached a maximum of 18.8%; the reaction time was prolonged, and the conversion rate of cyclohexene tended to balance, but Cyclohexanol yield decreased slightly. This is because prolonging the reaction time will increase the concentrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com