New maropitant crystal form and preparation method thereof
A maropy, amorphous technology, applied in organic chemical methods, separation/purification of carboxylic acid compounds, digestive system, etc. Problems such as poor water solubility and physicochemical stability, poor solubility of the free base crystal form of maropitant, etc., to achieve the effects of stable properties, good water solubility, and improved effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation of embodiment 1 amorphous maropitan citric acid
[0030] Dissolve 500.0 g of Maropitan free base (HPLC content 98.0%) and 224.0 g of citric acid monohydrate in 3.5 L of acetone, heat up to reflux to dissolve, heat filter, and add 3.5 L of methyl tert-butyl ether dropwise to the filtrate , stood at room temperature for crystallization overnight, filtered, and the filter cake was washed with methyl tert-butyl ether to obtain 688.0 g of crude product of maropitan citric acid monohydrate, with a yield of 95.0%.
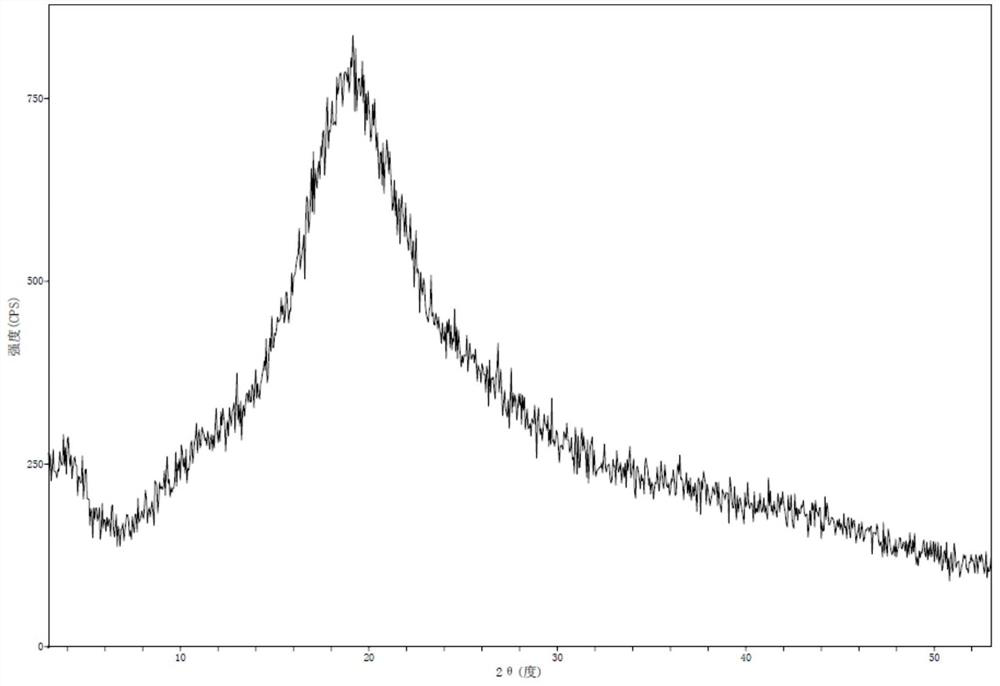
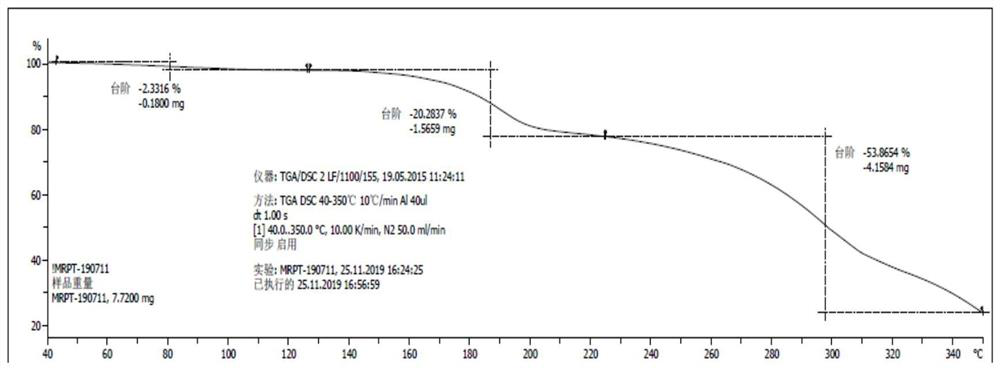
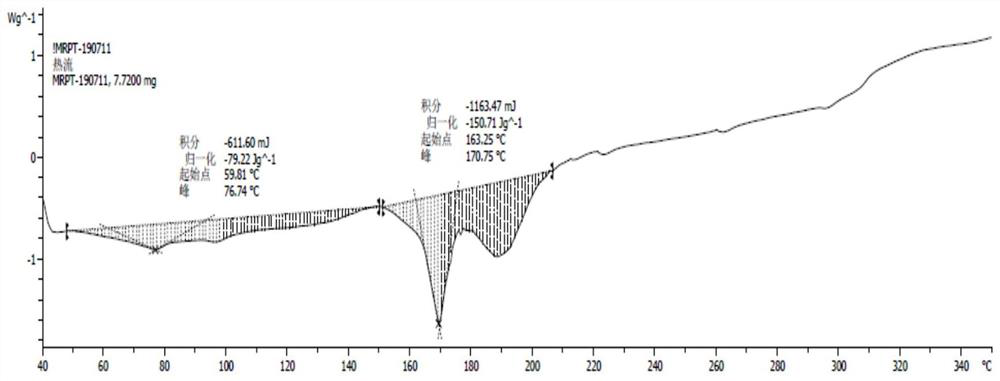
[0031] Add 688.0g of crude Maropitan citric acid monohydrate to 2.8L of acetone and heat up to 50°C to dissolve, filter, and concentrate the filtrate to dryness at 40°C under reduced pressure, and dry the obtained sample under reduced pressure at 70°C for 72 hours to obtain 685.7g of white powder Amorphous maropitan citric acid, yield 100%, HPLC content 100.10%, HPLC purity 99.91%.
[0032] Karl Fischer moisture detection method:
[0033] Accurate...
Embodiment 2
[0037] The preparation of embodiment 2 amorphous maropitan citric acid
[0038] Dissolve 500.0 g of Maropitan free base (HPLC content 98.0%) and 250.0 g of citric acid monohydrate in 2.5 L of acetone, heat up to reflux for 1 hour, heat filter, and add methyl tert-butyl ether dropwise to the filtrate 5.0 L, stand at room temperature for crystallization overnight, filter, and wash the filter cake with methyl tert-butyl ether to obtain 673.5 g of crude product of maropitan citric acid monohydrate, with a yield of 93.0%.
[0039] Add 673.5g of crude Maropitan citric acid monohydrate to 4.0L of acetone and heat up to 40°C to dissolve, filter, and concentrate the filtrate to dryness at 50°C under reduced pressure, and dry the obtained sample under reduced pressure at 75°C for 65 hours to obtain 671.2g of white powder Amorphous Maropitan citric acid, yield 100%, HPLC content 99.95%, HPLC purity 99.92%.
[0040] Karl Fischer moisture detection method:
[0041] Accurately weigh 0.2g ...
Embodiment 3
[0045] The preparation of embodiment 3 amorphous maropitan citric acid
[0046] Dissolve 500.0 g of Maropitan free base (HPLC content 98.0%) and 225.0 g of citric acid monohydrate in 5 L of acetone, heat up to reflux for 1 hour, heat filter, and add 5 L of methyl tert-butyl ether dropwise to the filtrate , stood at room temperature for crystallization overnight, filtered, and the filter cake was washed with methyl tert-butyl ether to obtain 681.0 g of crude product of maropitan citric acid monohydrate, with a yield of 94.0%.
[0047] Add 681.0 g of crude Maropitan citric acid monohydrate to 8.3 L of acetone and raise the temperature to 25°C to dissolve, filter, and concentrate the filtrate to dryness at 35°C under reduced pressure, and dry the obtained sample under reduced pressure at 60°C for 80 hours to obtain 678.7 g of white powder Amorphous Maropitan citric acid, yield 100%, HPLC content 99.92%, HPLC purity 99.85%.
[0048] Karl Fischer moisture detection method:
[004...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com