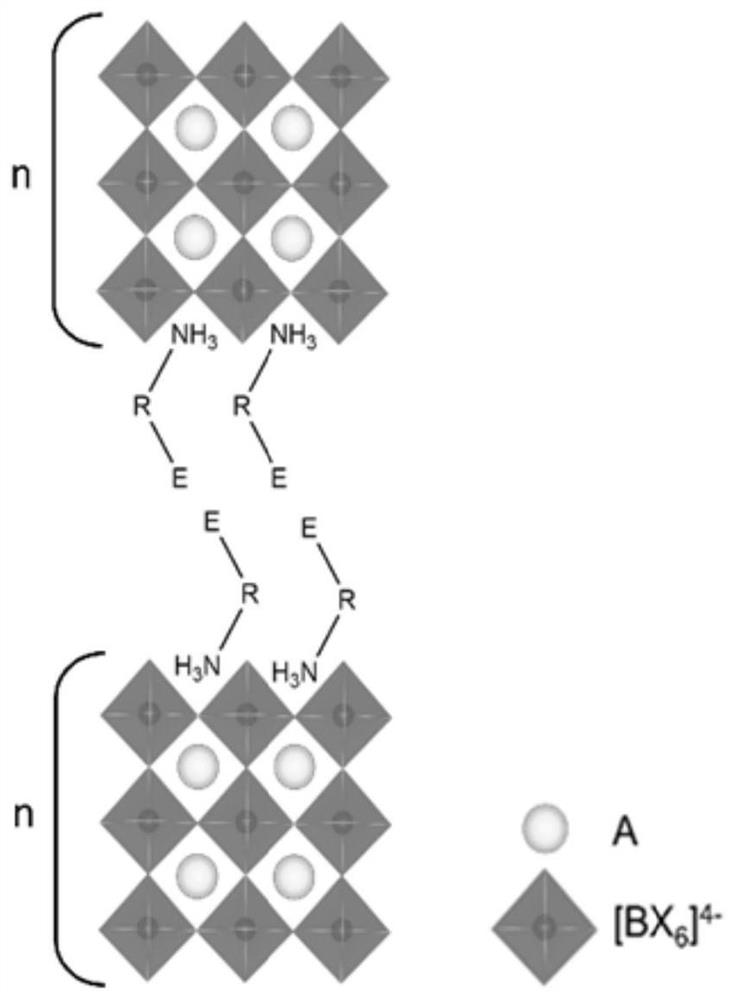
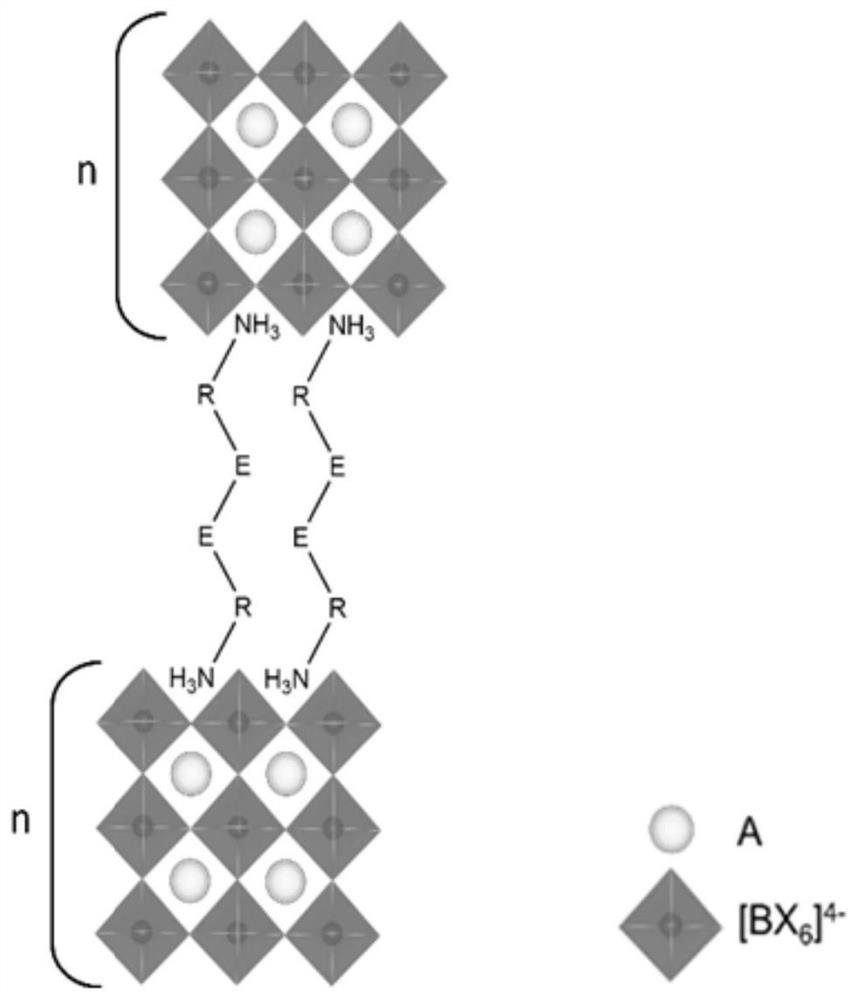
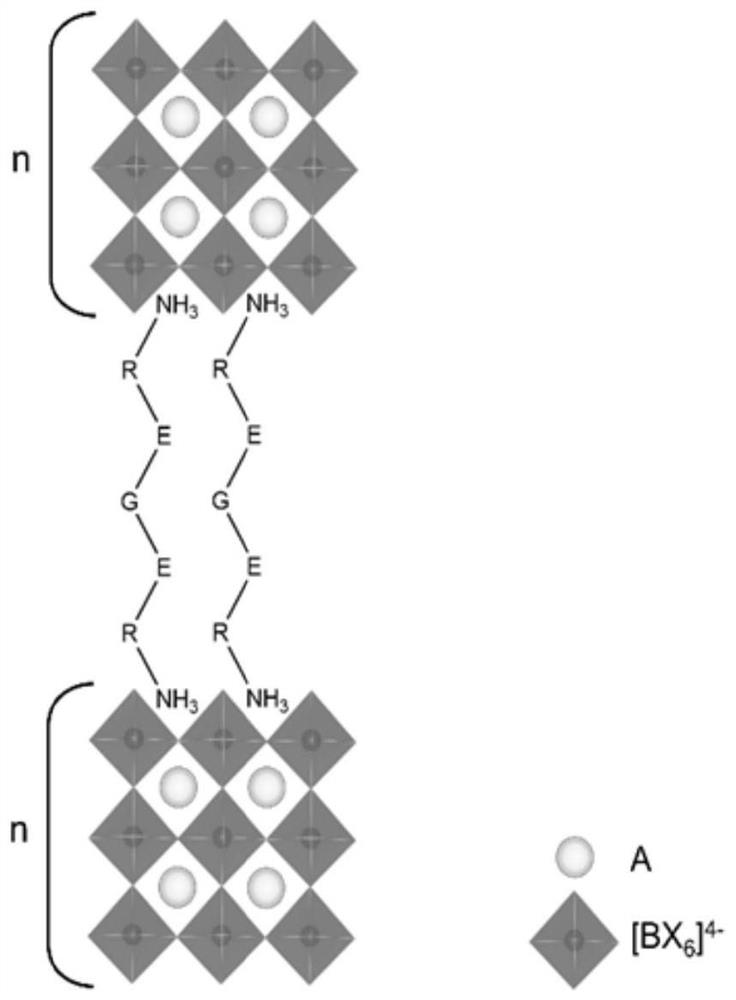
Dimension-reduced perovskite and its preparation method and application
A dimension-reduced perovskite and perovskite technology, applied in the field of dimension-reduced perovskite and its preparation, can solve problems such as limitations of preparation methods, and achieve the effect of strong controllability and high crystal conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] This embodiment has prepared (MEA) 2 PB 4 , the specific steps are:
[0080] S1: (PDSBA)PbI 4 Preparation of:
[0081] Add 0.5mmol lead iodide, 0.5mmol 2,2'-[propane-2,2-diylbis(thio)]diethylammonium iodate and 3mL hydroiodic acid into the reaction flask, heat at 100°C for half an hour until completely dissolved, then cool the reaction bottle naturally, take out the crystals after 1 day, and store them dry;
[0082] S2: (PDSBA)PbI 4 Convert to (MEA) 2 PB 4 Methods:
[0083] Add 20mg (PDSBA)PbI to the reaction vial 4 Crystal, 0.1mmol iodine element and 1mL dodecanethiol were stirred at room temperature, and the crystal was taken out after 1 day, and washed repeatedly with n-hexane to obtain (MEA) 2 PB 4 crystals.
[0084] The experimental results show that the (MEA) prepared in this embodiment 2 PB 4 , It is red under natural light, and emits green fluorescence under ultraviolet light, and its emission wavelength is 606nm.
[0085] (MEA) prepared in this em...
Embodiment 2
[0087] This embodiment has prepared (PDSBA)PbI 4 , the specific steps are:
[0088] S1: (MEA) 2 PB 4 Preparation of:
[0089] Add 0.2mmol lead iodide, 0.2mmol 2-aminoethanethiol hydrochloride, 1mL hydroiodic acid, 1mL hypophosphorous acid into the reaction flask, heat at 100°C for half an hour until completely dissolved, then transfer the reaction flask to minus 20°C Stand in the refrigerator, take out the crystals after 3 days, and store them dry;
[0090] S2: (MEA) 2 PB 4 Conversion to (PDSBA)PbI 4 Methods:
[0091] Add 20mg (MEA) to the reaction vial 2 PB 4 Put the crystal and 1mL of dodecanethiol in an acetone gas atmosphere, stir at room temperature, take out the crystal after 1 day, and wash it repeatedly with n-hexane to obtain (PDSBA)PbI 4 crystals.
[0092] Experimental result shows, the (PDSBA)PbI that present embodiment prepares 4 , It is orange under natural light, and emits green fluorescence under ultraviolet light, and its emission wavelength is 519n...
Embodiment 3
[0095] This embodiment has prepared (DSBA)PbI 4 , the specific steps are:
[0096] S1: (MEA) 2 PB 4 Preparation of:
[0097] Add 0.2mmol lead iodide, 0.2mmol 2-aminoethanethiol hydrochloride, 1mL hydroiodic acid, 1mL hypophosphorous acid into the reaction flask, heat at 100°C for half an hour until completely dissolved, then transfer the reaction flask to minus 20°C Stand in the refrigerator, take out the crystals after 3 days, and store them dry;
[0098] S2: (MEA) 2 PB 4 Conversion to (DSBA)PbI 4 Methods:
[0099] Add 20mg (MEA) to the reaction vial 2 PB 4 Crystal, 0.1mmol N-iodosuccinimide and 1mL tetrahydrofuran were stirred at room temperature. After 1 day, the crystal was taken out and washed repeatedly with tetrahydrofuran to obtain (DSBA)PbI 4 crystals.
[0100] Experimental result shows, the (DSBA)PbI that present embodiment prepares 4 , It is orange under natural light, and emits green fluorescence under ultraviolet light, and its emission wavelength is 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com