Ursolic acid pyrimidine amide derivative as well as preparation method and application thereof
A technology of ursolic acid pyrimidine amide and fruit acid pyrimidine amide, which is applied in the field of ursolic acid pyrimidine amide derivatives and its preparation, can solve problems such as threats to human health and difficulties in cancer treatment, and achieve novel structural effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
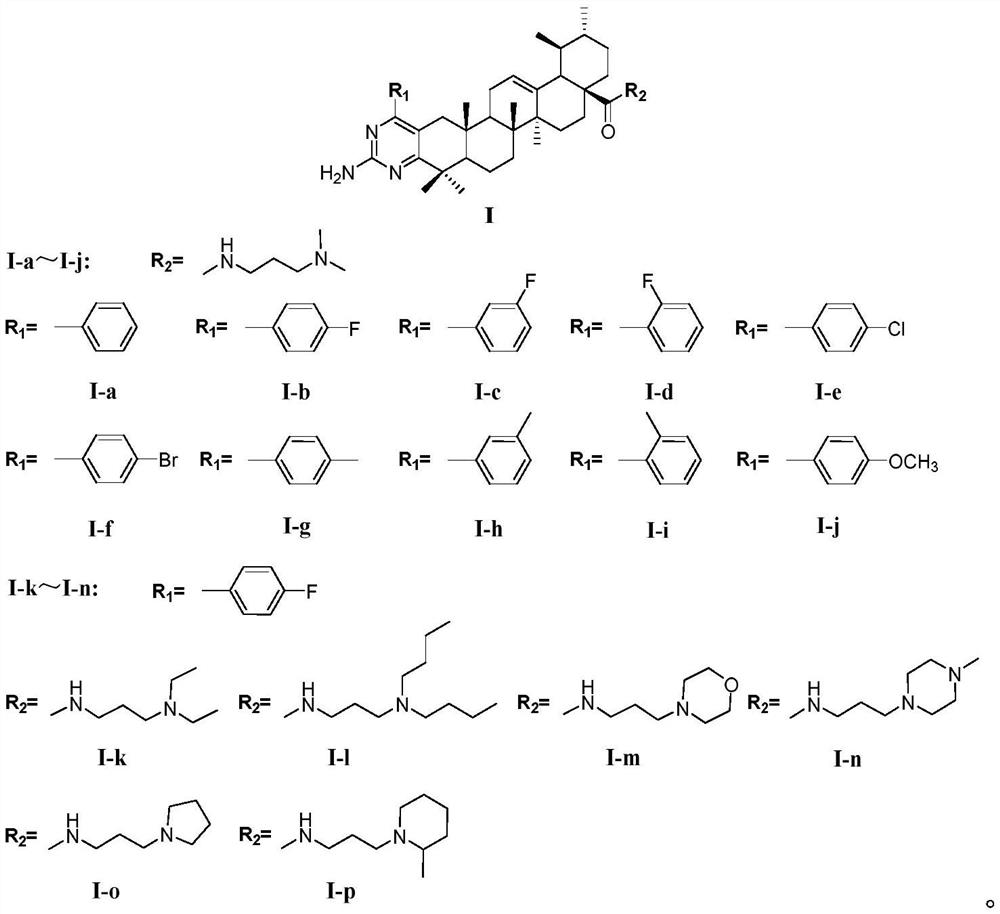
[0031] The preparation method of I-a to I-p of pyrimidine amide derivatives of ursolic acid comprises the steps:
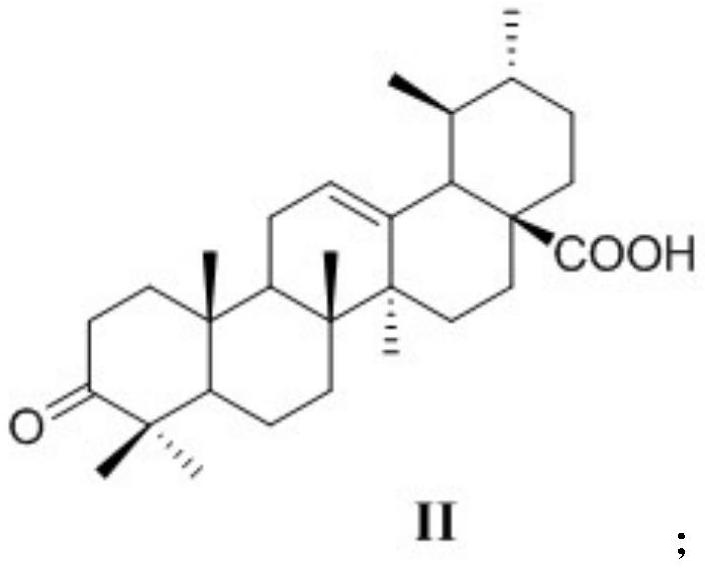
[0032] (1) ursolic acid obtains 3-oxidized ursolic acid through Jones reagent oxidation reaction, and structural formula is as shown in formula II:
[0033]
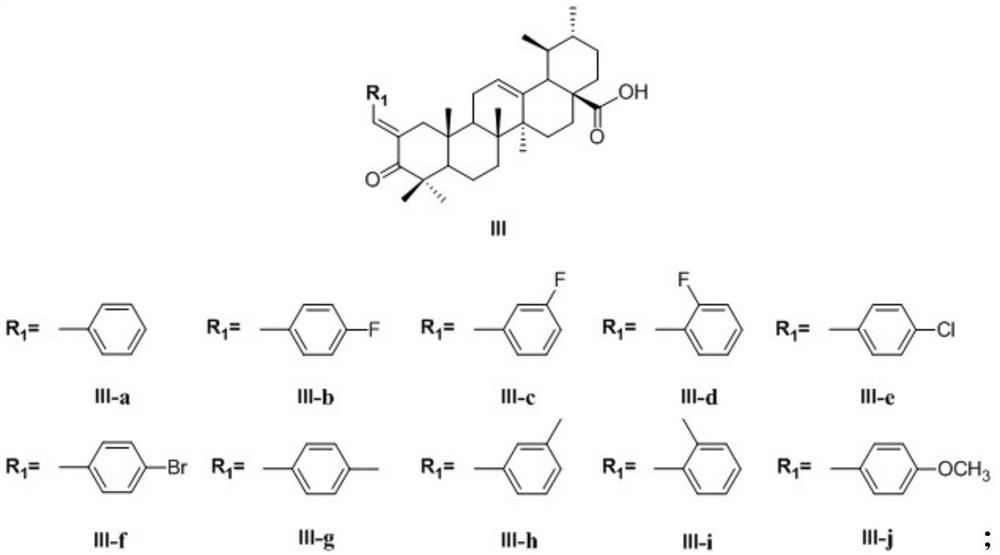
[0034] (2) Benzaldehyde of different substituents carries out ClaisenSchmidt condensation reaction with 3-oxidized ursolic acid under the effect of KOH / Ethanol to obtain corresponding benzylidene ursolic acids containing different substituents, and the structural formula is as shown in formula III:
[0035]
[0036] (3) The benzylidene ursolic acid III of different substituents obtains the amide compound of the corresponding benzylidene ursolic acid under the effect of DCC / HOBt, and the structural formula is as shown in formula IV:
[0037]
[0038] (4) Benzylidene ursolic acid amide compounds with different substituents react with guanidine hydrochloride under potassium tert-butoxide alkaline condi...
Embodiment 1
[0040]The synthesis of ursolic acid pyrimidine amide (I-a) comprises the following steps:
[0041] (1) Add 4.6mmol of ursolic acid and 250mL of acetone to a 500mL round bottom flask, stir to dissolve, stir in ice water for 15min, slowly add 1.87mL of Jones reagent dropwise and rise to room temperature, stir for 5h, then add 90mL of iso Propanol stirring reaction 30min, after the reaction finishes, filter and precipitate and collect the filtrate, the light yellow-green viscous solid obtained by concentrating the filtrate under reduced pressure obtains the white needle-shaped crystals obtained by methanol recrystallization, and makes 3-oxidized ursolic acid (II) ( 1.2g, 65.6%);
[0042] (2) Take by weighing 1.1 equivalents (relative to 3-oxidized ursolic acid) of benzaldehyde in a round bottom flask, add 20mL of absolute ethanol as a solvent, and take by weighing 0.5g of catalyst potassium hydroxide, and prepare a mass fraction of 2.5 % potassium hydroxide ethanolic solution, a...
Embodiment 2
[0047] Synthesis of Pyrimidinamide Ursolic Acid (I-b)
[0048] Using 4-fluorobenzaldehyde and 3-dimethylamino-1-propylamine as raw materials, prepare the benzylidene ursolic acid amide compound IV-b by the above method, and dissolve 0.001mol of compound IV-b in 10mL of tert-butyl Alcohol, slowly dissolve 0.005mol potassium tert-butoxide and 0.004mol guanidine hydrochloride in the reaction solution in turn, slowly rise to 85°C and stir and reflux for 24 hours. After the reaction is completed, extract the organic phase 3 times with ethyl acetate, wash 3 times with water, Wash once with saturated sodium bicarbonate solution and concentrated brine, remove water with anhydrous sodium sulfate, concentrate under reduced pressure to remove organic solvent to obtain a white solid, separate and purify it with an alumina column, and use dichloromethane / methanol as the gradient elution solvent (200:1-100:1), the product components were combined, concentrated under reduced pressure, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com