Preparation method of eribulin and intermediate thereof
A reaction system and compound technology, applied in the field of medicine, can solve the problems of high cost, difficult application, heavy metal pollution of the environment, etc., and achieve the effects of improving reaction yield, reducing chromium pollution, and being easy to purify
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
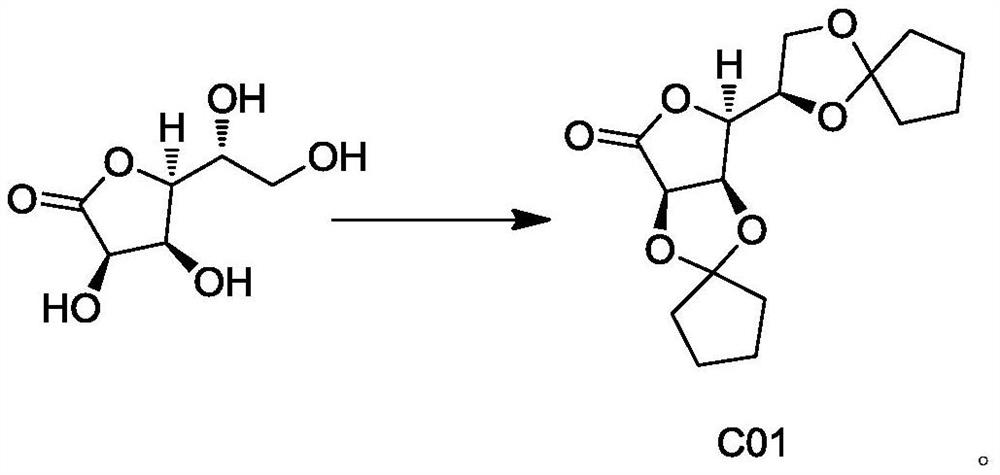
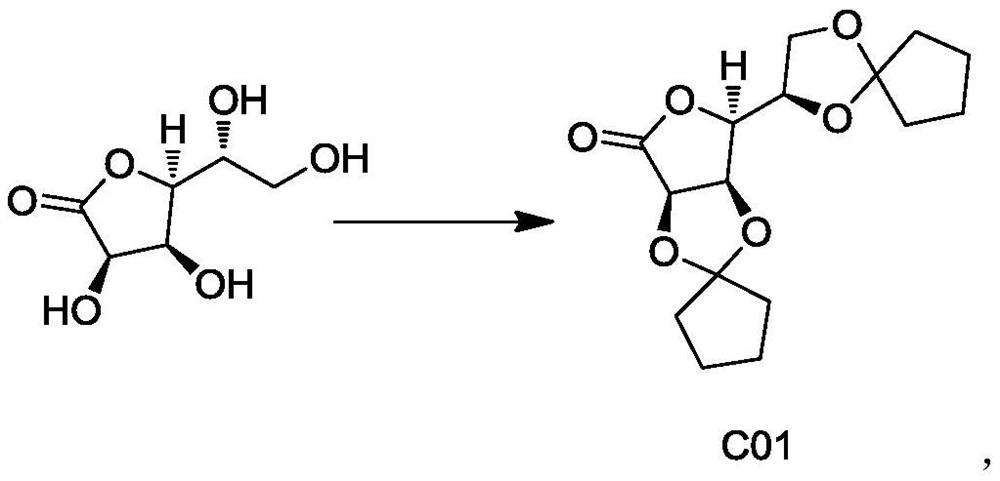
[0051] The preparation of embodiment 1 compound C01:
[0052]
[0053]0.11mol of D-gulonolactone, 0.35mol of p-methylcyclohexanone, 100ml of toluene and 0.03mol of anhydrous zinc chloride were stirred and refluxed. After the reaction, the reaction mixture was cooled, and 60ml of bicarbonate Sodium aqueous solution and 60ml saturated sodium chloride aqueous solution were washed. Concentrate the organic phase to remove part of the toluene, add 200ml of n-heptane, heat to reflux for 2-4 hours, cool to 40-50°C and stir for 2 hours. Filtration and drying yielded 34.14 g of compound C01.
Embodiment 2
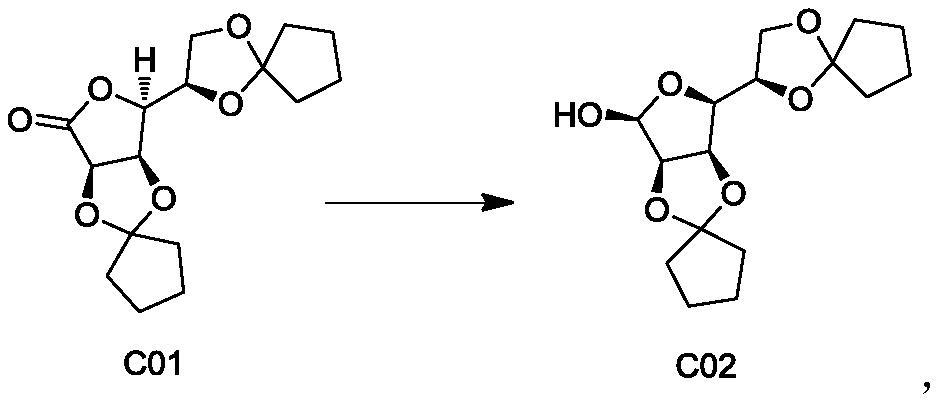
[0054] The preparation of embodiment 2 compound C02
[0055]
[0056] Dissolve 0.1mol of compound C01 in 250ml of toluene, cool to -10°C, slowly add 80ml of DIBALH (1.5M toluene solution), control the temperature not to exceed -10°C, stir for 30 minutes, add the reaction solution into potassium sodium tartrate solution, and the mixture to room temperature and stirred for 4 hours. The layers were separated by filtration, extracted twice with 60ml of ethyl acetate, the organic layers were combined, dried, filtered and concentrated to obtain 31g of compound C02.
Embodiment 3
[0057] The preparation of embodiment 3 compound C03
[0058]
[0059] 0.1 mol of compound C02 was dissolved in 80 ml of tetrahydrofuran, and 65 g of (methoxymethyl)triphenylphosphine chloride was added. At room temperature, 80 ml of tetrahydrofuran solution in which 36.5 g of NaHMDS was dissolved was slowly added dropwise. After the dropwise addition, the stirring was continued at room temperature. After the reaction was completed, 100 ml of brine, 100 ml of water, and 160 ml of ethyl acetate were added. Stir and separate layers, and slowly add 100 g of 20% sodium hydroxide solution to the organic phase under stirring. The organic layer was separated and concentrated to obtain the crude product of the compound represented by formula (C03). The crude product was stirred with 160ml of n-heptane and 20g of diatomaceous earth for 1 hour. Filter and wash the filter cake with 180 g of n-heptane. The filtrate was concentrated to 25% methanol solution and extracted three times w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com