Treatment method of human peripheral blood T lymphocytes, immunogen preparation and application
A technology of lymphocytes and human peripheral blood, applied in the direction of anti-animal/human immunoglobulin, cell culture active agent, blood/immune system cells, etc., can solve the problems of complex antigen components, poor standardization of titer testing, thymus Obtaining lymphocytes is difficult and other problems, to achieve the effect of simplifying the production and purification process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Separation, cryopreservation and quality evaluation of lymphocytes
[0024] Such as figure 1 As shown, take the leukocyte filter disc (acquisition source: Wuhan Central Blood Station) and place it in a biological safety cabinet, backflush the leukocyte filter disc with 0.9% normal saline, and collect the backflush normal saline containing leukocytes in a sterile bottle .
[0025] Use a pipette to draw 15mL of lymphocyte separation solution (manufacturer model: Fujian Sany) into the separation tube (50mL size), and add 15-30mL of diluted blood samples. Centrifuge at 1200g for 10min. After centrifugation, the sample in the separation tube is stratified from top to bottom: plasma-buffy coat-separation fluid-compartment-separation fluid-erythrocyte sedimentation. The sample stratification is as follows figure 2 shown. Use a pipette to suck the buffy coat into a new sterile centrifuge tube (50mL), add PBS buffer (pH≈7.2-7.4) to 45mL, resuspend the washed cell s...
Embodiment 2
[0031] Example 2 Proliferation culture conditions and surface antigen identification of lymphocytes
[0032] This embodiment explores the influence of culture conditions on in vitro proliferation and culture of lymphocytes, and studies lymphocyte proliferation medium (culture environment: 37 ° C, 5% CO 2 incubator) for cell proliferation cycle, state, density, activity and identification of surface antigen molecules. The exploration of some test cases is shown in Table 2:
[0033] Table 2 Statistical Table of Proliferation and Culture Exploration Test
[0034]
[0035]
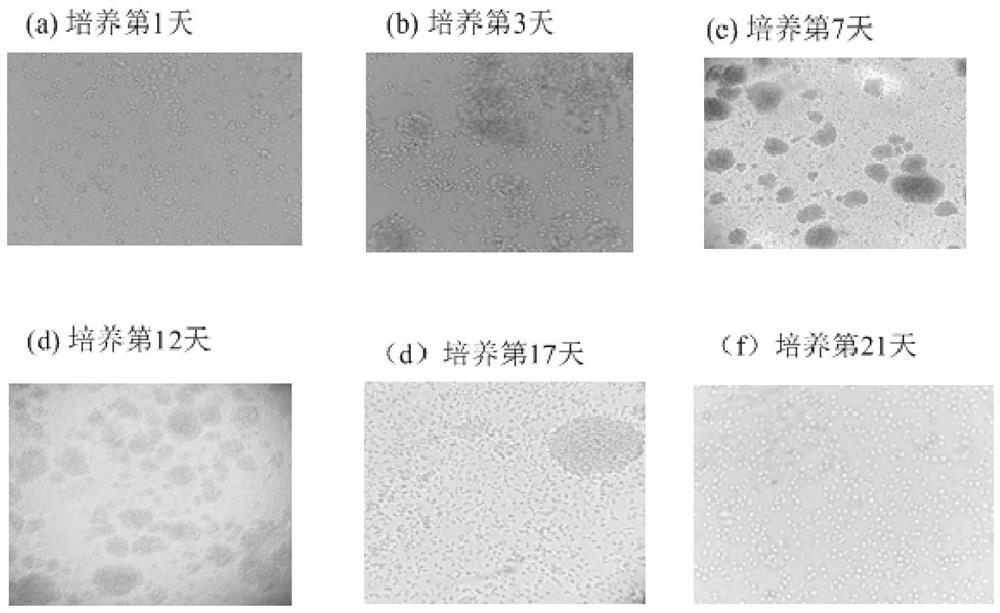
[0036] Among them, the culture conditions of the frozen cells in the test groups No. 2-6 are as follows: image 3 As shown, it shows that obvious lymphocyte aggregation can occur in 3 to 6 days, and the cell growth reaches a peak in 8 to 10 days of culture, and is in a plateau after 14 days (such as Figure 3-8 ), after 21 days, the cell viability detected by trypan blue staining was over 90%.
[003...
Embodiment 3
[0050] Example 3 Antigen preparation, pig immunization method and titer detection
[0051] The lymphocytes in the 2-6 test group that had been proliferated and cultured for 14 days were centrifuged at 1500 rpm for 5 minutes, collected, washed 2-3 times with 0.9% NaCl saline, and counted by a cell counter. The cell viability must be greater than 90%.
[0052] Prepare the amount of antigenic cells according to the weight of healthy pigs: 100 million proliferating lymphocytes for every 5-8 kg. Under aseptic conditions, take out the mineral oil adjuvant and the antigen and mix quickly according to the mass ratio of 1:1, then add 10 μL of molecular adjuvant according to the immune dose of each pig, mix well, shake for 10-20min, and form the oil-in-water antigen. Wherein, the formula for preparing the oil-in-antigen preparation is as follows in Table 5:
[0053] Table 5 The formula consumption table of different antigen preparations
[0054]
[0055] Multiple intramuscular inje...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com