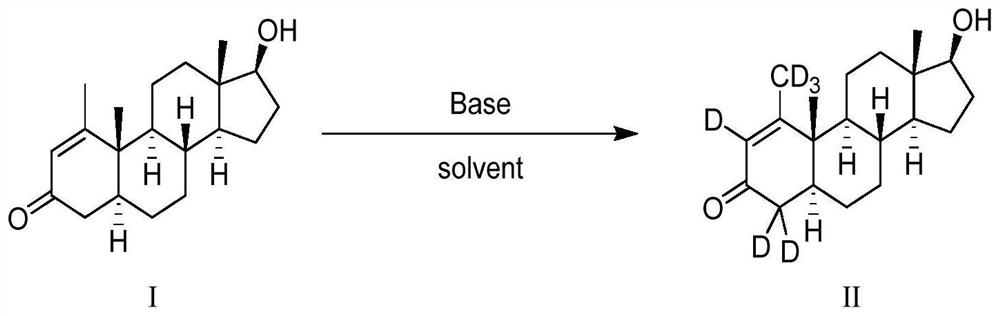
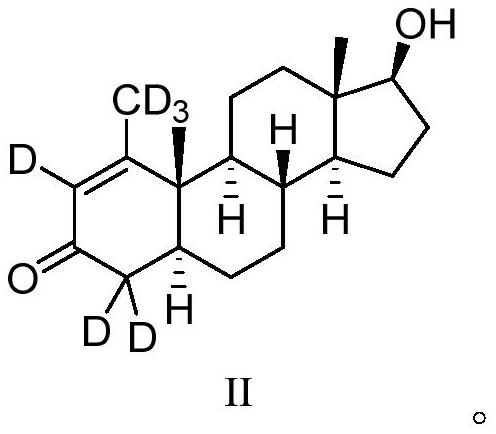
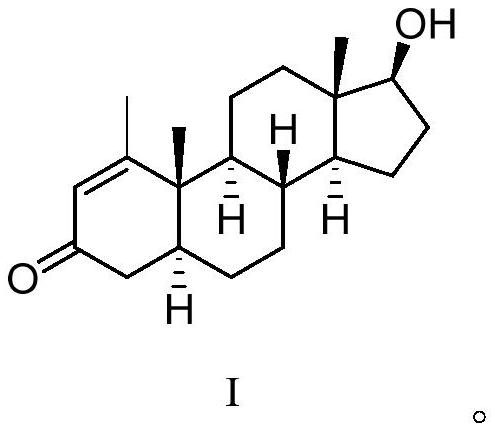
Deuterium-labeled metenolone stable isotope labeled compound
A technology of isotope labeling and primobolone, which is applied in the direction of isotope introduction into organic compounds, steroids, steroid isotope introduction, etc., can solve the problems of unsatisfactory internal standard reagents, danger, and little difference, etc., and achieve process design Reasonable, reproducible, and easy-to-operate results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The starting compound I (1.0 g) was dissolved in 13 mL of deuterated methanol (CH 3 OD), under nitrogen protection, sodium methoxide (0.5g, 3eq) was added at room temperature, and the reaction was carried out at this temperature for about 48 hours. The reaction mixture was quenched with 30% deuterium hydrochloric acid (DCl), monitored the system to pH = 6, extracted 2-3 times with dichloromethane, combined the organic phases, dried, filtered, and concentrated under reduced pressure to obtain the product, and repeated the above process 3 times , and then through normal phase purification to obtain the target compound (0.92g), the yield was 90%, the HPLC detection, the chemical purity was 99.3%, the MS detection, the isotope abundance was 94.7%.
Embodiment 2
[0033] The raw material compound I (1.5g) was dissolved in 10mL of tetrahydrofuran and 10mL of heavy water (D 2 O) under nitrogen protection, potassium carbonate (2.1g, 3eq) was added at room temperature, after the addition was complete, the reaction was carried out at this temperature for about 48 hours. The reaction mixture was quenched with 30% deuterium hydrochloric acid to pH = 6, extracted 2-3 times with ethyl acetate, the organic phases were combined, dried, filtered, and concentrated under reduced pressure to obtain a crude product. Repeat the above process 3 times, and then The target compound (1.4 g) was obtained by normal phase purification, with a yield of 92%, a chemical purity of 99.6% detected by HPLC, and an isotopic abundance of 93.0% detected by MS.
Embodiment 3
[0035] The raw material compound I (1.2g) was dissolved in 10mL of tetrahydrofuran and 10mL of heavy water (D 2 O) under nitrogen protection, sodium hydroxide (1.6g, 10eq) was added at room temperature, and after the addition was completed, the reaction was carried out at this temperature for about 48 hours. The reaction mixture was quenched with 30% deuterated hydrochloric acid to pH = 6, extracted 2-3 times with acetic acid, the organic phases were combined, dried, filtered, and concentrated under reduced pressure to obtain a crude product. Repeat the above process 3 times for normal phase purification The target compound (1.1 g) was obtained with a yield of 91.0%, a purity of 99.1% detected by HPLC, and an isotopic abundance of 92.5% detected by MS.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com