Method for measuring content of lactoferrin in infant milk powder
A determination method and technology for lactoferrin, which can be applied to measurement devices, instruments, scientific instruments, etc., can solve problems such as being unsuitable for lactoferrin, and achieve the effects of simple method, good repeatability and high detection efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] S1: Weigh 5.0g of infant milk powder to be measured into a 50mL centrifuge tube, add 30mL of phosphate buffer A to the centrifuge tube, and vortex to completely dissolve the infant milk powder in phosphate buffer A. Phosphoric acid solution is then used to adjust the pH to 4.5 to precipitate impurity proteins in the infant milk powder to obtain a mixed solution. Phosphate buffer A can use 0.1mol / L NaH 2 PO 4 -Na 2 HPO 4 buffered saline solution.
[0029] S2: Centrifuge the mixed solution obtained in S1 for 10 min at a centrifugation speed of 10000 r / min to completely separate the solid-liquid phase in the mixed solution. Transfer the supernatant obtained after centrifugation to a 50 mL volumetric flask, use phosphate buffer A to dilute the supernatant to the mark, and mix and shake well. After being filtered through a filter membrane, the sample solution was obtained.
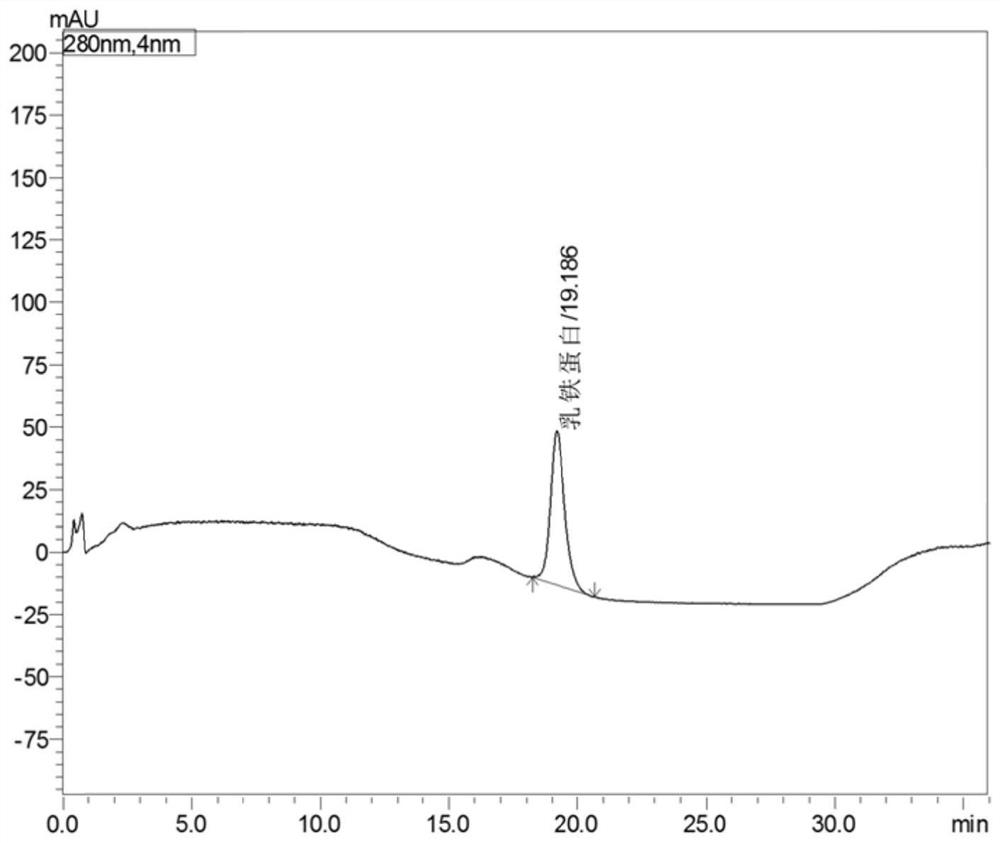
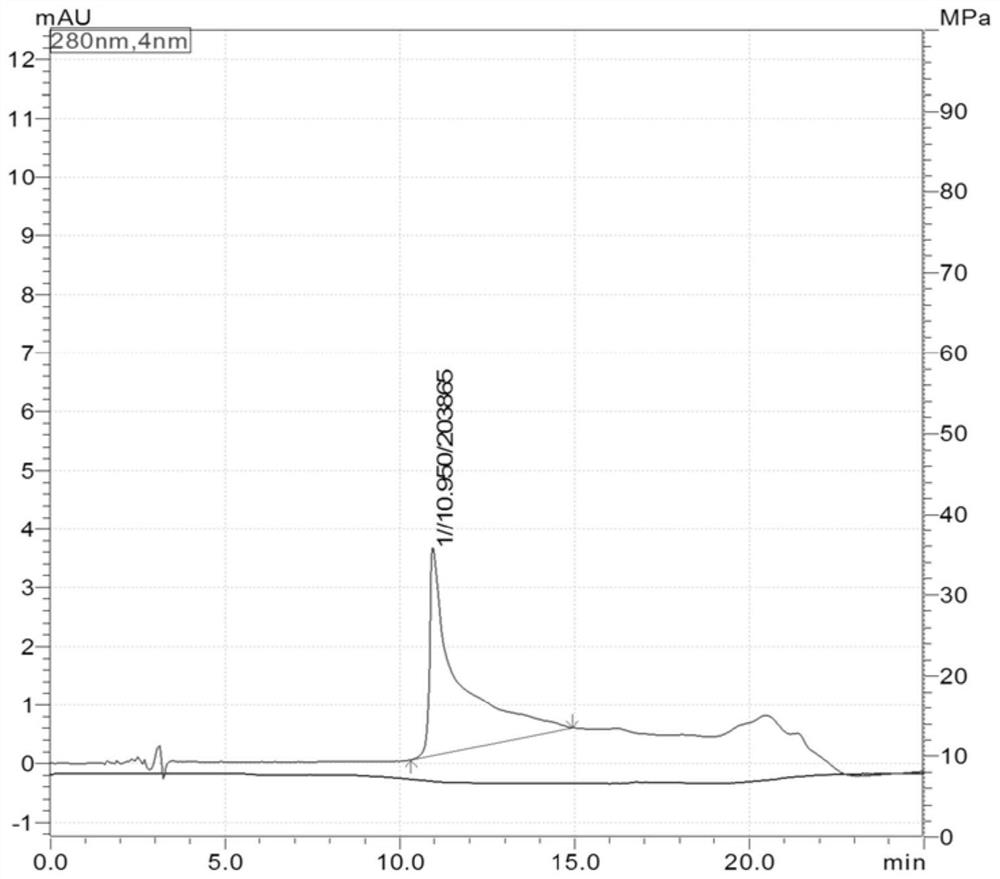
[0030] S3: After filtering the sample solution, take 2mL of the sample solution for detection b...
Embodiment 2
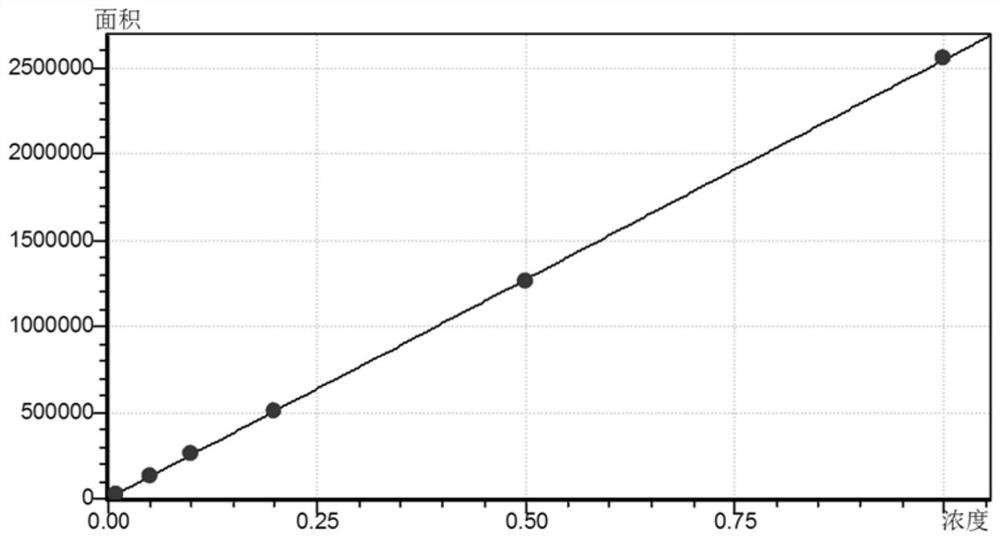
[0036] 1. Take the peak area y of the collection of illustrative plates in embodiment 1 as the ordinate, and the lactoferrin standard solution mass concentration x (mg / mL) as the abscissa, draw a standard curve and carry out linear regression. See the standard curve image 3 , as can be seen from the figure, within the concentration range of 0.01-1.0 mg / mL, the linear relationship between the obtained peak area and concentration is relatively good. The linear regression equation of lactoferrin is y=2546380x-640.579, and the correlation coefficient is 0.99998.
[0037] Using the method in Example 1, the 0.5 mg / mL lactoferrin standard solution was repeatedly injected 6 times, and the lactoferrin peak area was determined, as shown in the table below. The calculated relative standard deviation (RSD) is 1.46%, and the RSD is lower than 5.00%. It can be seen from the results that the precision is good and the stability of the instrument is good.
[0038]
[0039] 2. Take the same...
Embodiment 3
[0044] 1. Add different concentrations of lactoferrin to the blank milk powder, and carry out the standard recovery test. Using the method in Example 1, each group was subjected to 6 parallel experiments, and the results were obtained in the following table. It can be seen from the table that the standard addition recovery rate is 89.9-101%, and the relative standard deviation is 1.61-4.00%.
[0045]
[0046] It can be seen that the relative standard deviation of the assay method of the present invention is small and the standard addition recovery rate is high.
[0047] 2. Using the method in Example 1, different brands of infant milk powder on the market were tested, and the test results are shown in the table below.
[0048]
[0049] It can be seen from the results that the content of lactoferrin in different brands of infant milk powder is different. The nutritional content table shows that the milk powder with exogenous lactoferrin is detected, but the nutritional c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com