Spinal cord needle biopsy tissue fixation decalcification solution, preparation method and decalcification method
A technique of puncture biopsy tissue and preparation method, which is applied in the field of pathological detection, can solve the problems affecting the reliability of immunohistochemical detection and molecular pathological detection results, hidden dangers of environment and operator safety and security, and has no tissue fixation effect, and achieves staining. The effect is better, the decalcification time is shortened, and the effect of meeting the requirements of the pathological diagnosis report
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
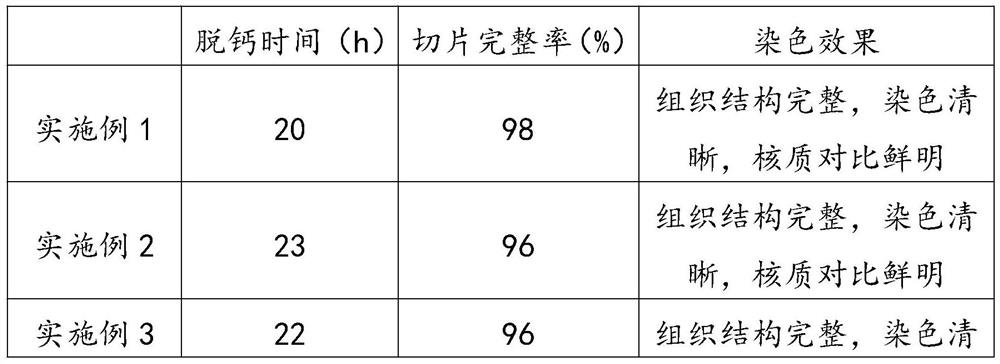
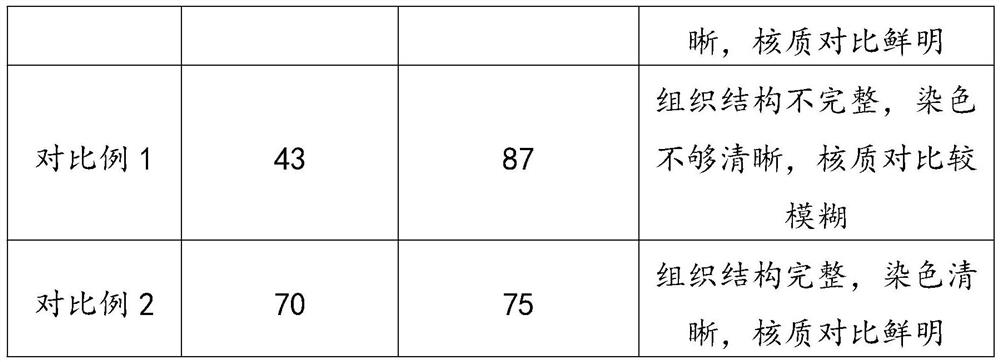
[0031] A solution for fixing and decalcifying spinal cord biopsy tissue, which is made of the following ingredients in parts by weight: 32 parts of formaldehyde, 350 parts of methanol, 450 parts of saturated ethylenediaminetetraacetic acid solution, 8 parts of PBS buffer solution, 30 parts of accelerator, chlorinated 2 parts of sodium, 128 parts of water.
[0032] The preparation method of described EDTA saturated liquid is as follows:
[0033] (1) Mix ethylenediaminetetraacetic acid and water at a mass ratio of 13:100 and stir for 10 minutes;
[0034] (2) Send the above mixed solution into a constant temperature box and keep it at 60°C for 10 hours;
[0035] (3) Take it out and store it at room temperature.
[0036] The accelerator is prepared by mixing aluminum chloride, chloroform, and polyethylene glycol octylphenyl ether in a mass ratio of 17:11:2.
[0037] The preparation method of described spinal cord biopsy tissue fixation decalcification solution comprises the fol...
Embodiment 2
[0046] A spinal cord biopsy tissue fixation decalcification solution, which is made of the following ingredients in parts by weight: 30 parts of formaldehyde, 300 parts of methanol, 400 parts of ethylenediaminetetraacetic acid saturated solution, 7 parts of PBS buffer solution, 25 parts of accelerator, chlorinated 1 part sodium, 100 parts water.
[0047] The preparation method of described EDTA saturated liquid is as follows:
[0048] (1) Mix ethylenediaminetetraacetic acid and water at a mass ratio of 13:100 and stir for 10 minutes;
[0049] (2) Send the above mixed solution into a constant temperature box and keep it at 60°C for 10 hours;
[0050] (3) Take it out and store it at room temperature.
[0051] The accelerator is prepared by mixing aluminum chloride, chloroform, and polyethylene glycol octylphenyl ether in a mass ratio of 17:11:2.
[0052] The preparation method of described spinal cord biopsy tissue fixation decalcification solution comprises the following steps...
Embodiment 3
[0061] A spinal cord biopsy tissue fixation decalcification solution, which is made of the following ingredients in parts by weight: 35 parts of formaldehyde, 400 parts of methanol, 500 parts of ethylenediaminetetraacetic acid saturated solution, 9 parts of PBS buffer solution, 35 parts of accelerator, chlorinated 3 parts of sodium, 150 parts of water.
[0062] The preparation method of described EDTA saturated liquid is as follows:
[0063] (1) Mix ethylenediaminetetraacetic acid and water at a mass ratio of 13:100 and stir for 10 minutes;
[0064] (2) Send the above mixed solution into a constant temperature box and keep it at 60°C for 10 hours;
[0065] (3) Take it out and store it at room temperature.
[0066] The accelerator is prepared by mixing aluminum chloride, chloroform, and polyethylene glycol octylphenyl ether in a mass ratio of 17:11:2.
[0067] The preparation method of described spinal cord biopsy tissue fixation decalcification solution comprises the follow...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com