Method for synthesizing aromatic difluoroboron beta-diketonate compounds by one-pot process
A technology for diketone boron difluoride and aromatic compounds, which is applied in the field of one-pot synthesis of aryl-β-diketone boron difluoride compounds, can solve the problems of high cost and low yield, and achieve raw material cost Low, high yield and purity, simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] In a three-necked flask equipped with a thermometer, add 1.08g of anisole, 5.2g of acetic anhydride, and 1.41g of boron trifluoride ether, stir magnetically and heat the oil bath to 100°C, keep it warm for 2h, then let it stand to cool to room temperature, add Dilute with ethyl acetate-petroleum ether mixed solvent (the volume ratio of ethyl acetate and petroleum ether is 2:1), filter out the insoluble solid, and concentrate the filtrate for recrystallization (recrystallization solvent is ethyl acetate-petroleum ether mixed solvent, acetic acid The volume ratio of ethyl ester and petroleum ether is 1:1) to separate out a yellow solid to obtain the target product [2,2-difluoro-6-(4-methoxyphenyl)-4-methoxybenzene substituted Base-2H-1,3,2-dioxaboron complex] with a yield of 82% and a purity of 97%.
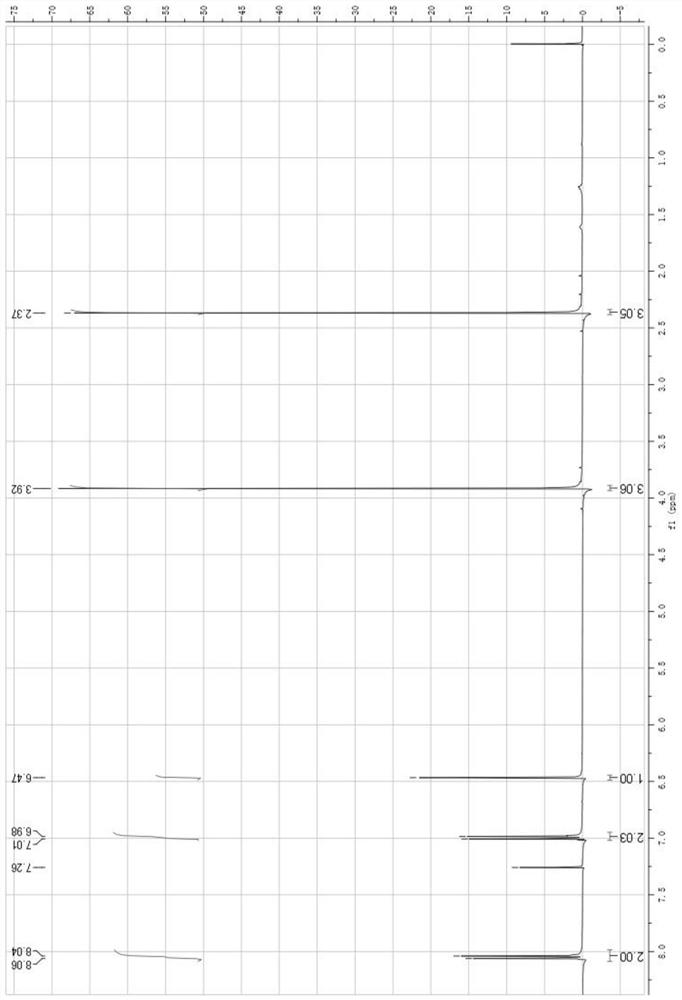
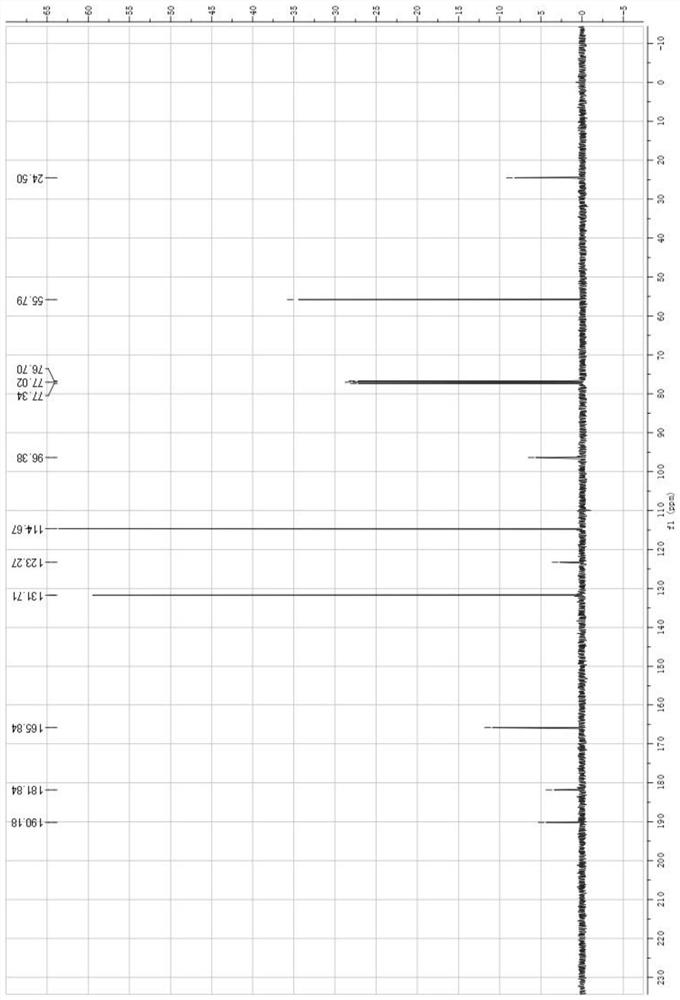
[0058] For NMR characterization see figure 1 with figure 2 , the spectrogram data are as follows:
[0059] 1 HNMR:CDCl 3 , 2.37 (s, 3H); 3.92 (s, 3H); 6.47 (s, 1H); 6....
Embodiment 2
[0062] In a three-necked flask equipped with a thermometer, add 0.84 g of thiophene, 5.2 g of acetic anhydride, and 1.41 g of boron trifluoride ether, stir magnetically and heat in an oil bath to 100°C, keep it warm for 2 hours, then let it stand to cool to room temperature, and add ethyl acetate Ester-petroleum ether mixed solvent (the volume ratio of ethyl acetate and petroleum ether is 2:1) is diluted, and the insoluble solid is filtered out, and the filtrate is concentrated and purified by column chromatography (the solvent used for purification is ethyl acetate-petroleum ether mixed solvent , the volume ratio of ethyl acetate and petroleum ether is 1:1), to obtain the thiophene-substituted target product [2,2-difluoro-4-methyl-6-(thiophen-2-yl)-2H-1,3 , 2-Dioxyboron complex], yield 60%, purity 98%.
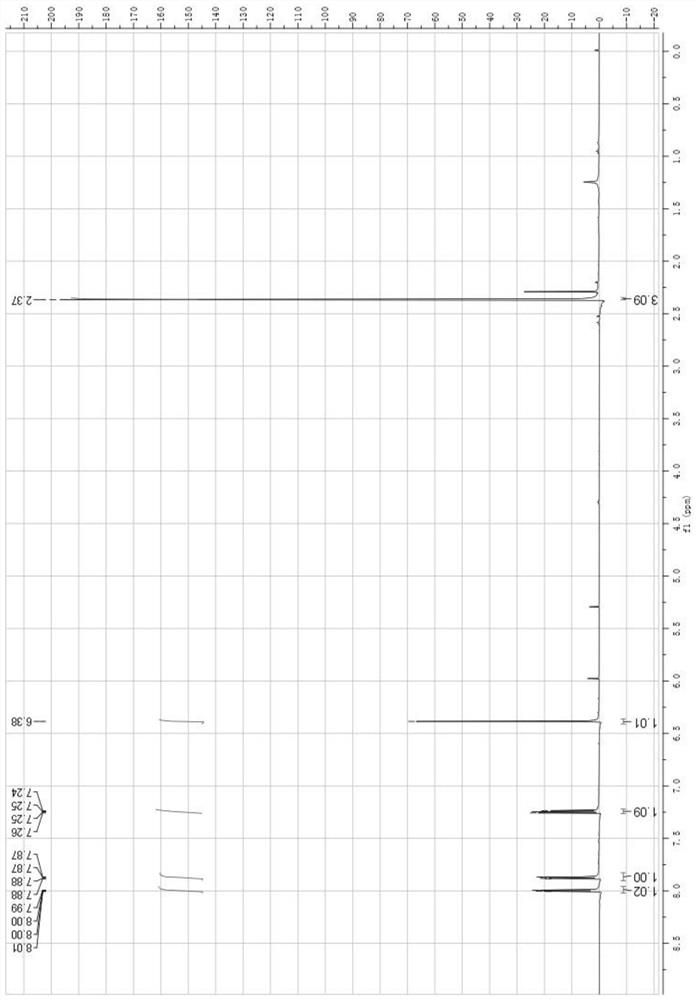
[0063] See attached picture for NMR characterization image 3 with Figure 4 , the spectrogram data are as follows:
[0064] 1 HNMR:CDCl 3 , 2.37 (s, 3H); 6.38 (s, 1H); ...
Embodiment 3
[0067] In a three-necked flask equipped with a thermometer, add 1.38g of m-xylylene dimethyl ether, 5.2g of acetic anhydride, and 1.41g of boron trifluoride ether, stir magnetically and heat to 100°C in an oil bath, keep it warm for 2h, then let it stand and cool to room temperature , adding ethyl acetate-petroleum ether mixed solvent (the volume ratio of ethyl acetate and petroleum ether is 2:1) to dilute, filter out the insoluble solid, and the filtrate is concentrated and purified by column chromatography (the solvent used for purification is ethyl acetate-petroleum ether Ether mixed solvent, the volume ratio of ethyl acetate and petroleum ether is 1:1), to obtain the target product [6-(2,4-dimethoxyphenyl)-2,2-dimethoxybenzene substituted Fluoro-4-methyl-2H-1,3,2-dioxaboron complex], yield 70%, purity 98%.
[0068] See attached picture for NMR characterization Figure 5 with Image 6 , the spectrogram data are as follows:
[0069] 1 HNMR:CDCl 3 ,2.33(s,3H);3.91(s,3H);...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com