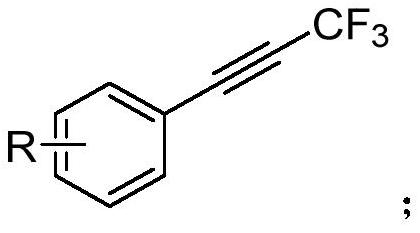
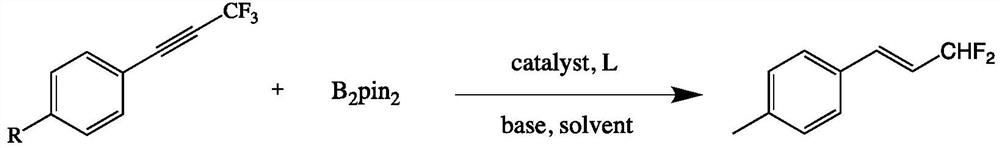
Synthesis method of 1,1-difluoro-2-propylene olefin compound
A synthetic method and technology of propylene hydrocarbons, applied in the preparation of organic compounds, carbon-based compounds, chemical instruments and methods, etc., to achieve the effects of less by-products, high yield, and wide applicability of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]
[0028] To a 25 mL high-pressure nitrogen storage equipped with a magnetic stirrer, add raw materials 1-methyl-4-(3,3,3-trifluoro-1-propynyl)benzene (0.2 mmol), cuprous iodide (0.02 mmol), potassium tert-butyl alkoxide (0.3mmol), B 2 Pin 2(pinacol diboronate) (0.6mmol), tricyclohexylphosphine (PCy3) (0.02mmol), add 2.0mL toluene under argon protection; fix the reaction tube on a magnetic stirrer, and react the mixture at 90°C for 4h Afterwards, the reaction ends; add an appropriate amount of water to the reaction solution, extract with ethyl acetate, dry over anhydrous sodium sulfate, and finally use rotary evaporation to remove the solvent, and the crude product is separated and purified by column chromatography (eluent is petroleum ether) to obtain The target product (2a), yield 60%. The NMR data of this compound are: 1 H NMR (600MHz, CDCl3) δ7.35 (d, J = 7.9Hz, 2H), 7.19 (d, J = 7.9Hz, 2H), 6.87–6.84 (m, 1H), 6.34–6.15 (m, 2H) ,2.38(s,3H). 13 CNMR(151MHz, CD...
Embodiment 2
[0030]
[0031] Substitute 1-methyl-3-(3,3,3-trifluoro-1-propynyl)benzene (1b) for 1-methyl-4-(3,3,3-trifluoro-1-propyne Base) benzene (1a), others are with embodiment 1. Column chromatography (petroleum ether: ethyl acetate = 100:1) gave the target product (2b) in a yield of 67%. The NMR data of this compound are: 1 H NMR (400MHz, CDCl 3 )δ7.30–7.17(m,3H),7.18–7.17(m,1H),6.89–6.83(m,1H),6.40–6.10(m,2H),2.38(s,3H). 13 C NMR (101MHz, CDCl 3 )δ138.61, 137.38(t, J=12.2Hz), 134.52, 130.34, 128.83, 128.05, 124.56, 120.90(t, J=24.0Hz), 115.62(t, J=233.5Hz), 21.45. 19 F NMR (376MHz, CDCl 3 )δ-109.31–-109.50(m,2F).
Embodiment 3
[0033]
[0034] Replace 1-methyl-4-(3,3,3-trifluoro-1-propane) with 1-tert-butyl-4-(3,3,3-trifluoro-1-propynyl)benzene (1c) Alkynyl) benzene (1a), others are the same as in Example 1. Column chromatography (petroleum ether: ethyl acetate = 100:1) gave the target product (2c) in a yield of 70%. The NMR data of this compound are: 1 H NMR (400MHz, CDCl 3 )δ7.42–7.38(m,4H),6.90–6.84(m,1H),6.39–6.10(m,2H),1.34(s,9H). 13 C NMR (101MHz, CDCl 3 )δ152.92, 137.09(t, J=12.3Hz), 131.81, 127.18, 125.90, 120.31(t, J=24.0Hz), 115.76(t, J=233.3Hz), 34.90, 31.35. 19 F NMR (376MHz, CDCl 3 )δ-109.05–-109.23(m,2F).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com