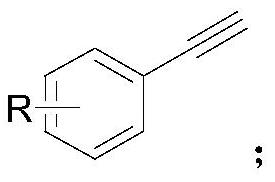
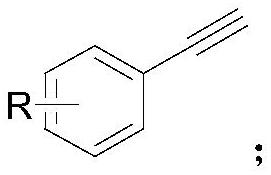
Synthesis method of alkenyl borate compound
A kind of technology of alkenyl borate ester and synthesis method, applied in the field of organic synthesis, achieves the effects of high yield, low content of harmful metals, easy and safe operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]
[0036] Put neopentylglycol diboronate (0.30mmol, 1.5equiv) and 4-dimethylaminopyridine (0.60mmol, 3.0equiv) into a transparent Schlenk tube equipped with a stirring bar, vacuumize the tube, and then blow in argon Air, repeat 3-4 times. Add biphenylacetylene (1a) (0.20mmol, 1.0equiv), Pd(PCy 3 ) 2 Cl 2 (400ppm) and solvent (ethanol, 3mL), the reaction mixture was stirred at 70°C for 10 hours; after the reaction was completed, it was cooled to room temperature, quenched with water and extracted with ethyl acetate, the organic layers were combined, dried over sodium sulfate, and then concentrated in vacuo , the product was purified by silica gel flash column chromatography (petroleum ether / ethyl acetate=20:1) to give colorless oil (2a) (44mg, 75%). The NMR data of this compound are: 1 H NMR (600MHz, CDCl 3 ) δ7.59(d, J=8.1Hz, 2H), 7.56(d, J=2.6Hz, 3H), 7.43(t, J=7.7Hz, 2H), 7.37(d, J=18.3Hz, 1H) ,7.33(t,J=7.4Hz,1H),6.14(d,J=18.3Hz,1H),3.71(s,4H),1.01(s,6H). 13 ...
Embodiment 2
[0038]
[0039] Table 1 Low-concentration metal-catalyzed hydroboration of alkynes
[0040]
[0041]
[0042] For the above reaction, we used 4-ethynyl-1,1'-biphenyl (1) and neopentyl glycol diboronate (2) as model substrates, o-xylene as solvent, and potassium acetate as base. A suitable metal catalyst concentration of 400ppm is used to realize the hydroboration reaction of alkynes. Attempts to start cheap metals, 400ppm (0.04mol%) nickel chloride, or its combination with various phosphine ligands (PPh 3 ,PCy 3 , Xantphos) can also provide a lower yield of the target product, but more by-products. The combination of 400ppm (0.04mol%) cuprous chloride and phenanthroline ligand (1,10-Ph, NC) cannot provide the target product, and the combination of 400ppm (0.04mol%) cuprous chloride and phosphine ligand (PPh 3 ,PCy 3 , Sphos, Xphos, 5mol%) can provide a lower yield of the target product, but the reaction is more complicated. Excitingly, 400ppm of Pd(PCy 3 ) 2 Cl...
Embodiment 3
[0044]
[0045] The optimization of the base of table 2 alkyne hydroboration
[0046] Entry Base Yield(%) b
[0047] After screening for suitable catalysts, bases were screened. Three equivalents of sodium acetate provided 50% of the target product. Sodium carbonate, potassium carbonate, cesium carbonate can provide the target product (25%, 30%, 15%) of lower yield, potassium hydroxide can provide 33% target product, and sodium hydroxide can not provide target product either. Organic bases were also investigated, with triethylamine providing 22% of the target product and 1,8-diazabicyclo[5.4.0]undec-7-ene providing 55% of the target product. Triethylenediamine can provide 10% of the target product, and 4-dimethylaminopyridine has the best effect, which can provide 65% of the target product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com