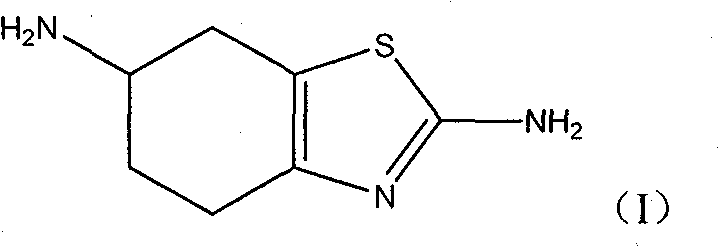
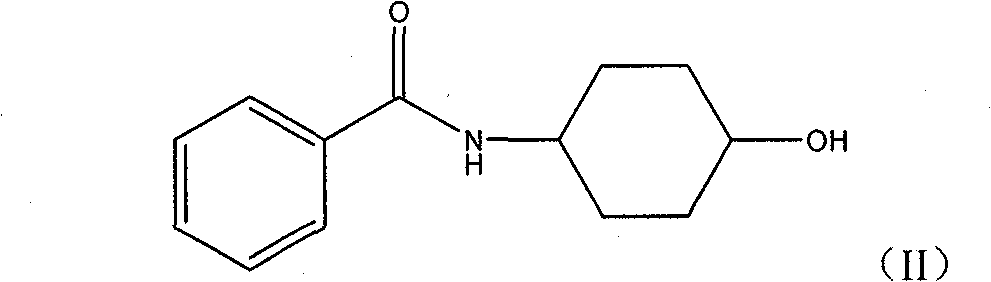
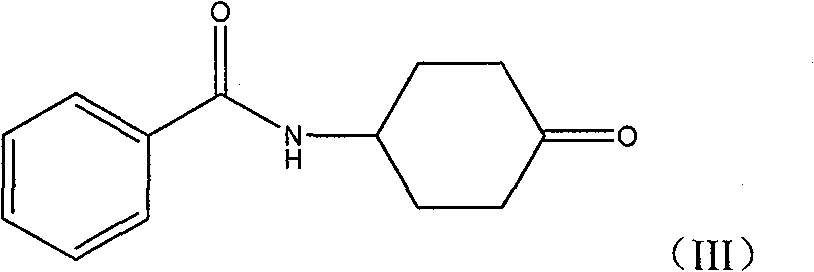
Process for producing pramipexole midbody 2,6-diamino-4,5,6,7-tetrahydrochysene-benzothiazole
A technology of benzothiazole and diamino, applied in the field of chemistry, can solve the problems of high cost, difficult removal, and low process yield, and achieve the effects of mild reaction time, reduced separation steps, and flexible and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Preparation of 4-Benzamidocyclohexanol. Dissolve 203.60 g (1.767 mol) of trans-4-aminocyclohexanol in 1000 ml of pyridine. The reaction liquid was heated to 110° C. with an oil bath, and 400.00 g (1.770 mol) of benzoic anhydride was added to the reaction liquid. Continue to stir for 3 hours after the reaction liquid is clarified, pour the reaction liquid into a beaker and stand for crystallization, filter and separate the product 4-benzamidocyclohexanol, and dry to obtain 356.00 g of white product with a yield of 91.9%.
Embodiment 2
[0050] Preparation of 4-Benzamidocyclohexanone. Dissolve 61ml of concentrated sulfuric acid in 270ml of water, then add 62.00g of chromium trioxide to obtain an oxidizing solution, and cool it for later use. Dissolve 160.00 g (0.730 mol) of 4-benzamidocyclohexanol in a mixed solution of 1000 ml glacial acetic acid and 1000 ml chloroform, and stir until clear. Then the prepared oxidizing solution (aqueous sulfuric acid solution of chromium trioxide) is dropped into the reaction solution, and the reaction temperature is strictly controlled between 25°C and 35°C. After the dropwise addition, the stirring reaction was continued for 3 hours. 160ml of water was added to the reaction solution, stirred thoroughly, and then the chloroform layer was separated. The aqueous layer was extracted twice with 200 ml of chloroform. The chloroform layers were combined, washed with 800 ml of water, the chloroform layer was separated, dried over anhydrous magnesium sulfate, filtered and distill...
Embodiment 3
[0052] Preparation of 2,6-diamino-4,5,6,7-tetrahydro-benzothiazole. 236.0 g (1.087 mol) of 4-benzamidocyclohexanone were dissolved in 500 ml HAc and 125 ml HBr. Under the conditions of avoiding light and cooling in an ice-water bath, and keeping the temperature between 5 °C and 15 °C, 177.28 g (1.108 mol) of bromine was added dropwise, and the reaction was continued for 1.5 h with stirring after dropping. At this time, 165.0 g (2.17 mol) of thiourea was added, the mixture was heated to 90° C., and refluxed for 3 h. 200ml of 20% hydrochloric acid was added, and the reaction was refluxed for 24h. Then the reaction solution was cooled to about 10°C, neutralized with a saturated KOH solution, and kept in an ice-water bath. The product 2,6-diamino-4,5,6,7-tetrahydro-benzothiazole was isolated by washing with cold water followed by drying. 104.2 g of a white product was obtained, with a yield of 57.2%.
[0053] The obtained 2,6-diamino-4,5,6,7-tetrahydro-benzothiazole can be res...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com