<18>F-labeled EGFR positron imaging agent as well as preparation method and application thereof
A semi-preparation and reaction technology, applied in the field of 18F-labeled EGFR positron imaging agent and its preparation, to achieve fast clearance in vivo, good specificity, and good tracer effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
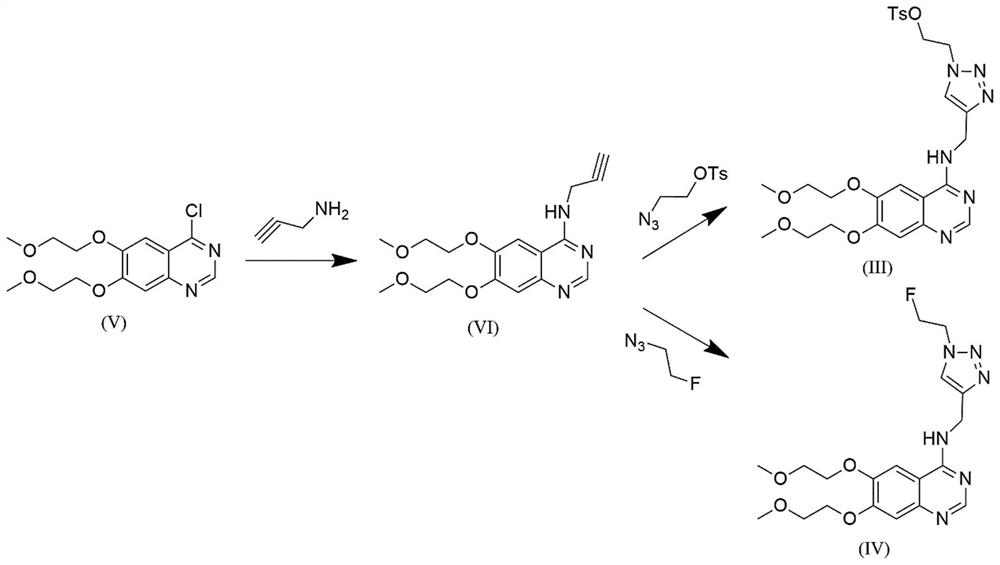
[0087] The compound shown in embodiment 1 formula (III) ( 18 Preparation method of F-labeled EGFR positron imaging agent precursor)
[0088] 4-Chloro-6,7-bis(2-methoxyethoxy)quinazoline (formula (V), 40.00g, 1eq), DMF (N,N-dimethylformazol Amide, 200mL), potassium carbonate (35.38g, 2eq), propargylamine (8.48g, 1.2eq), stirred at 90°C for 2.5h. After the reaction was monitored by TLC (thin-layer chromatography), the temperature was lowered to room temperature (20-30° C.), and 400 mL of water was added and stirred evenly. Filter and wash twice with 400 mL of water. The filter cake was spin-dried to obtain 24.5 g of a silver-white solid product (formula (VI)), with a yield of 57.8%.
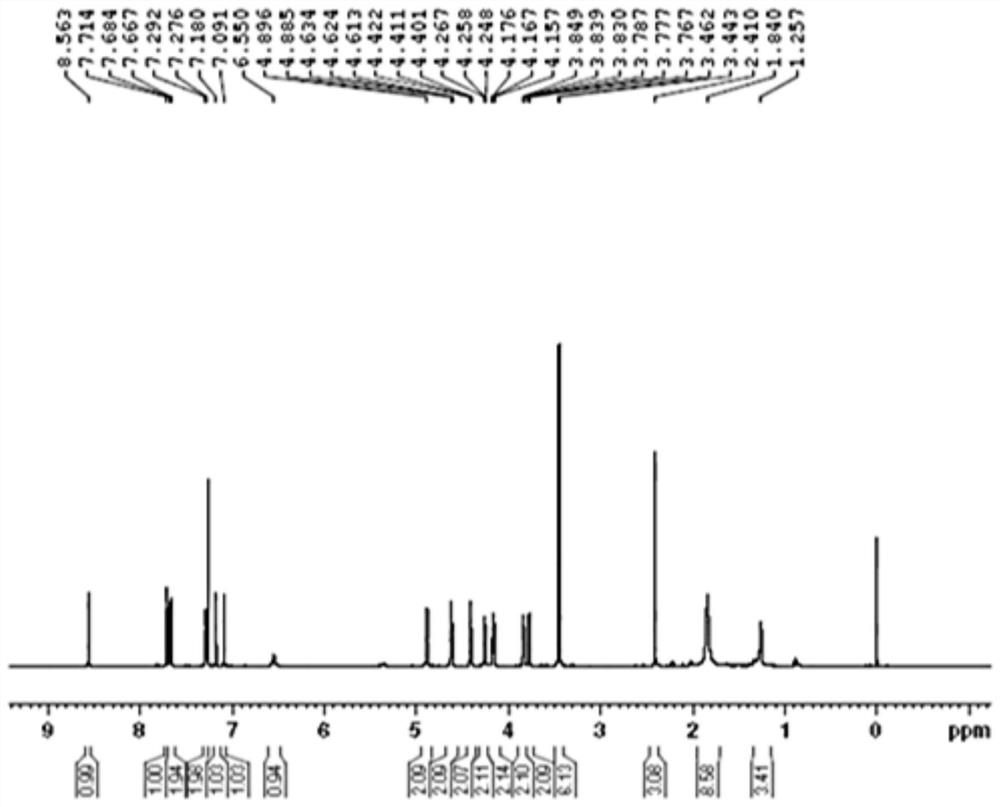
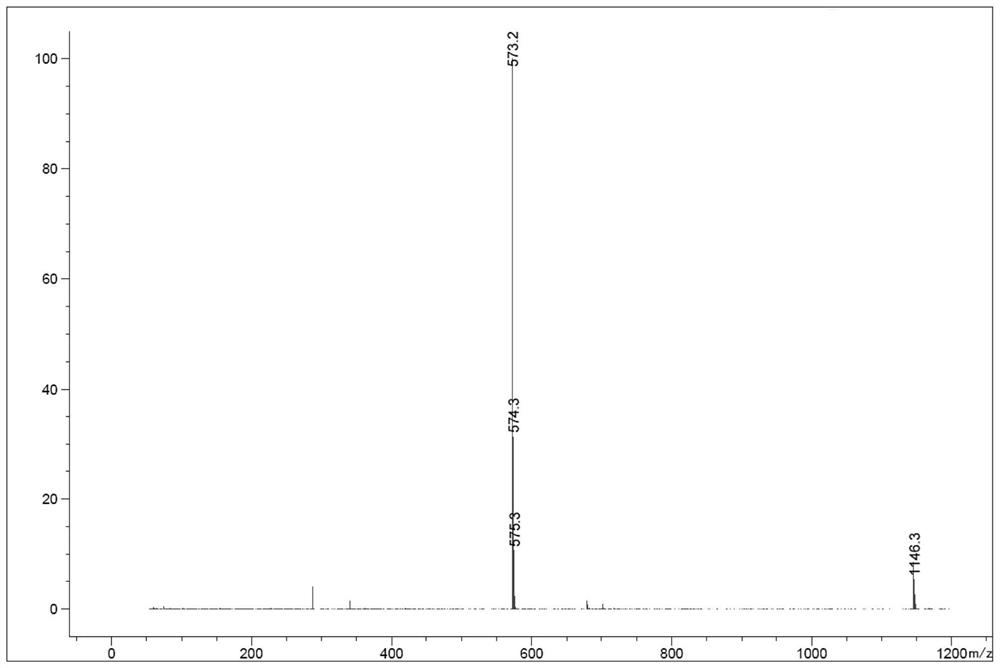
[0089] In the reaction flask, add 4-propargylamino-6,7-bis(2-methoxyethoxy)quinazoline (formula (VI), 0.42g, 1.0eq), copper sulfate pentahydrate (0.018g , 0.05eq), sodium erythorbate (0.026g, 0.1eq), 2mL each of water and tert-butanol, then add azidoethyl toluenesulfonate (0.332g, 1.0eq), and s...
Embodiment 2
[0091] The compound shown in embodiment 2 formula (IV) ( 18 The preparation method of F-marked EGFR positron imaging agent standard substance)
[0092] 4-Chloro-6,7-bis(2-methoxyethoxy)quinazoline (formula (V), 40.00g, 1eq), DMF (N,N-dimethylformazol Amide, 200mL, 5V), potassium carbonate (35.38g, 2eq), propargylamine (8.48g, 1.2eq), stirred at 90°C for 2.5h. After TLC (thin-layer chromatography) monitors the completion of the reaction, the temperature is lowered to room temperature (20-30° C.), and 400 mL (10 V) of water is added and stirred evenly. Filter and wash twice with 400ml of water. The filter cake was spin-dried to obtain 24.5 g of a silver-white solid product (formula (VI)), with a yield of 57.8%.
[0093] In the reaction flask, add 4-propargylamino-6,7-bis(2-methoxyethoxy)quinazoline (formula (VI), 2.70g, 1.0eq), copper sulfate pentahydrate (0.40g , 0.2eq), sodium ascorbate (1.78g, 1.1eq), water and DCM (dichloromethane) each 27mL, then added 2-fluoroethane az...
Embodiment 3
[0095] The compound shown in embodiment 3 formula (II) ( 18 F-marked EGFR positron imaging agent) preparation method
[0096] (1) Use a medical cyclotron to bombard H 2 18 O, pass 18 O(p,n) 18 F nuclear reaction produces 500mCi 18 F, and conduct in anion exchange column, measure activity and use 1.5mL mixed solution (15.0mg 4,7,13,16,21,24-hexaoxo-1,10-diazabicyclo[8.8.8]20 Hexane (K 2.2.2. ) plus 4.5mg K 2 CO 3 dissolved in 0.15mL water and 1.35mL acetonitrile) will 18 F is rinsed into the reaction bottle;
[0097] (2) Continuously blow high-purity helium into the reaction bottle, azeotropically remove water at 110°C for 3 minutes, and dry; dissolve 5 mg of precursor in 1 mL of anhydrous DMSO solution, add to the reaction bottle, and react at 110°C for 15 minutes.
[0098] (3) After cooling the reaction solution, carry out semi-preparative HPLC separation to obtain 18 F-labeled EGFR positron imaging agent, the separation conditions are: the chromatographic column is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com