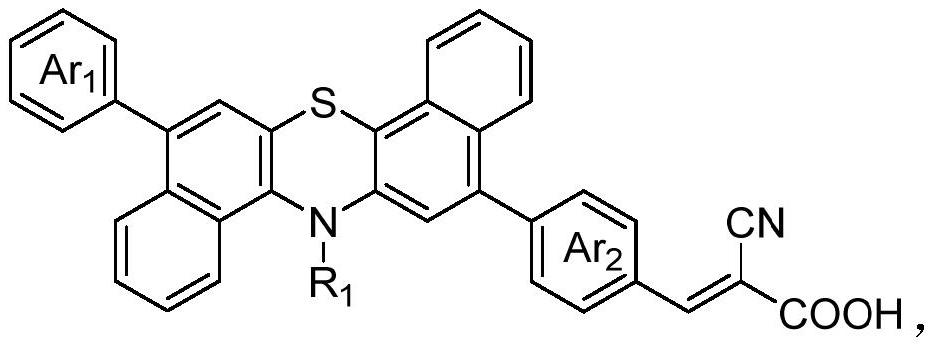
Organic dye sensitizer based on dibenzophenothiazine as well as preparation method and application of organic dye sensitizer
A technology of benzophenothiazine and organic dyes, which is applied in the field of organic photoelectric functional materials, can solve the problems of complex synthesis, toxicity, and low yield, and achieve the effects of common and easy-to-obtain raw materials, low production costs, and high absorption coefficient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Use hexyl chain to modify triphenylamine as donor, phenyl is the preparation method of dibenzophenothiazine-based organic dye molecule P1 of π spacer, the synthetic route of described preparation method is as follows:
[0039]
[0040] The preparation method comprises the following steps:
[0041] 1. Preparation of compound 1: under a nitrogen atmosphere, put 1,2'-dinaphthylamine (1.35g, 5mmol), sulfur (0.32g, 5mmol) and iodine (0.33g, 1.3mmol) in a 250ml round bottom In the flask, 30 ml of o-dichlorobenzene as a solvent was added, and the reaction mixture was raised to 180° C., kept at this temperature for 30 minutes, and then cooled to room temperature. After the reaction was complete, the dark reaction mass was dissolved in a small amount of acetone and enough petroleum ether was added to produce two layers. After separation of the layers, the dark solution was extracted several times with fresh warm petroleum ether and the petroleum ether layers were combined. ...
Embodiment 2
[0048] Modified phenyl carbazole with hexyl chain as donor, thiophene is the preparation method of dibenzophenothiazine-based organic dye molecule P2 of π spacer, the synthetic route of described preparation method is as follows:
[0049]
[0050] The preparation method comprises the following steps:
[0051] The synthesis steps of compounds 1, 2, and 3 are the same as those in Example 1.
[0052] Preparation of compound 4b: under nitrogen atmosphere, compound 3 (541 mg, 1 mmol), (5-formylthiophen-2-yl) boronic acid (180 mg, 1.2 mmol), K 2 CO 3 (345mg, 2.5mmol), Pd[P(C 6 h 5 ) 3 ] 4 (50mg, 0.05mmol) and TBAB (96.6mg, 0.3mmol) were placed in a three-necked flask, poured into 15ml of tetrahydrofuran solvent, heated to 85°C and refluxed for 13h. When the latter should be complete, add water to quench the reaction. After the reaction solution was cooled to room temperature, it was extracted 3 times with DCM and brine, and dried with NaSO4. The crude product was then pur...
Embodiment 3
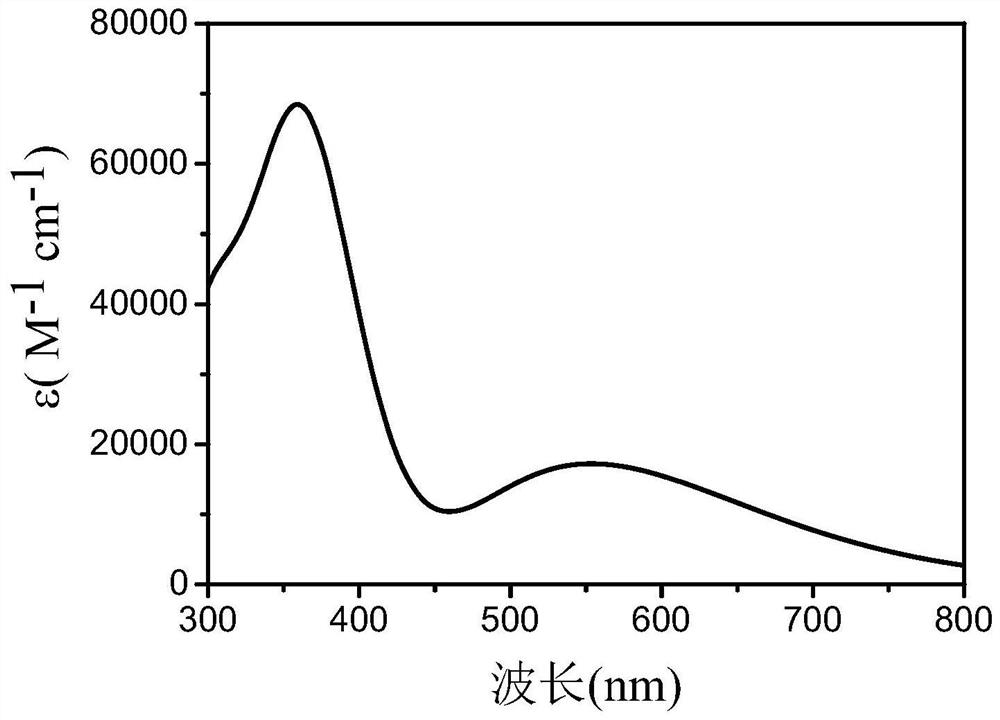
[0056] The absorption spectrum of the P1 dye: the organic dye with hexyl chain modified triphenylamine as the electron donor, dibenzophenothiazine as the core group of the π bridge, and the benzene ring as the π spacer have a maximum absorption peak of ultraviolet-visible light at about 560nm. When the molar absorptivity (ε) can reach up to 6.8×10 4 L·mol -1 cm -1 , see figure 1 . The sensitizer prepared by the invention has stable structure, proper energy gap and high absorption coefficient.
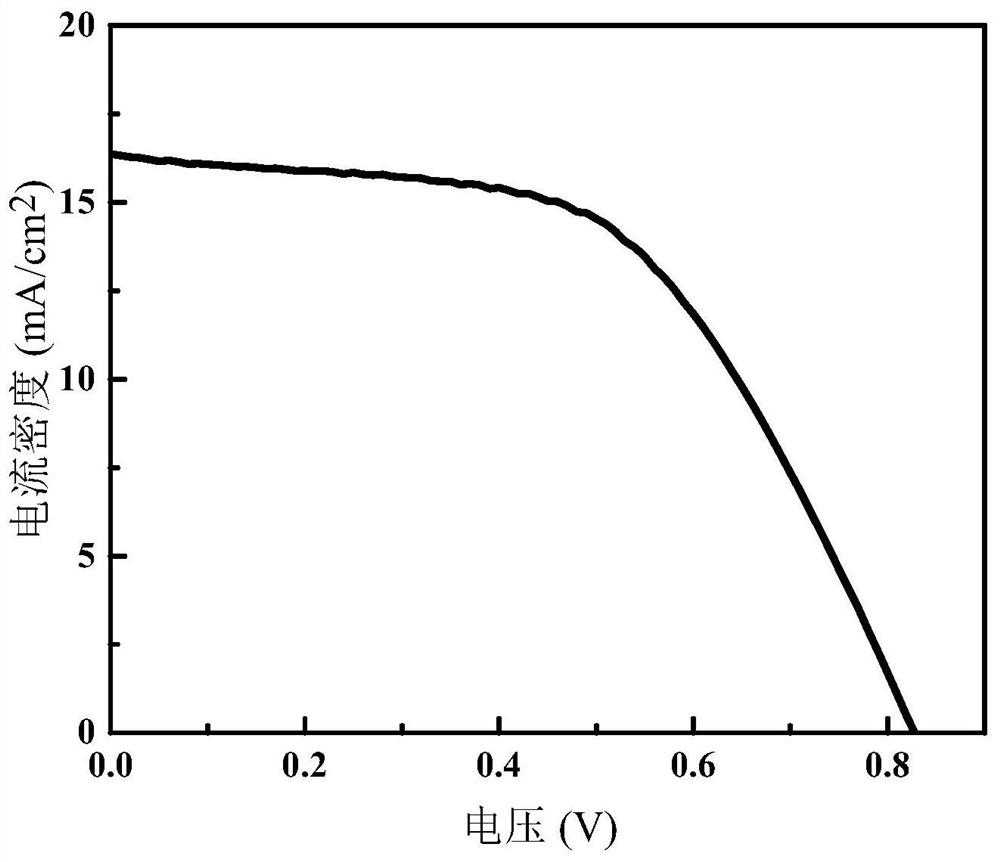
[0057] The preparation method of the sensitized solar cell based on P1 dye comprises the following steps: the FTO substrate is coated with a detergent on the surface and scrubbed, washed with water and then placed on a film rack, and then the film rack is put into a beaker, and used in turn. Ionized water, absolute ethanol, and acetone were sonicated for 15 minutes each. After the end, the cleaned substrate was taken out and dried in an oven at 110°C for 1 hour. The conductive surfa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com