Ursolic acid derivative capable of protecting gastric mucosa and preparation method thereof
A technology of ursolic acid and derivatives, applied in the field of chemical drug synthesis, can solve problems such as damage to gastric mucosa, unfavorable transdermal administration, gastrointestinal irritation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
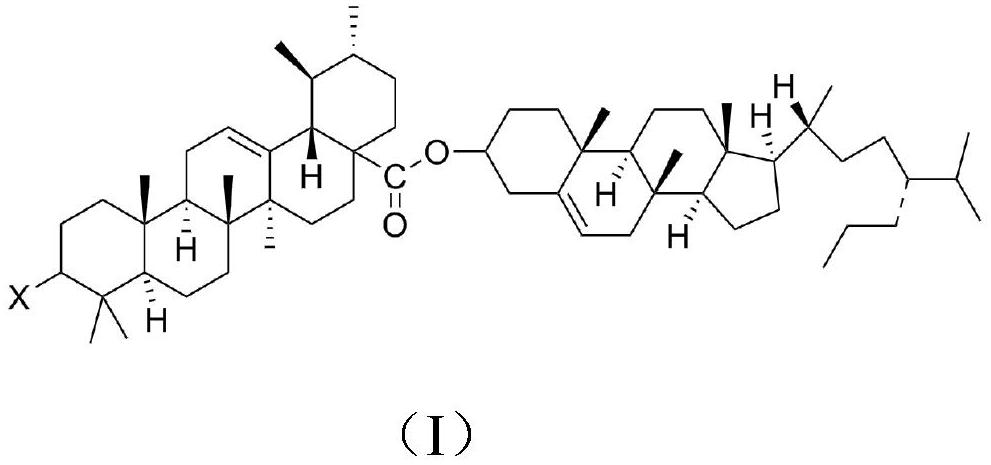
[0026] (1) Dissolve 20g of ursolic acid in 60mL of N,N-dimethylformamide, add 5.2g of acetyltriphenylphosphorus chloride, heat to 40°C, react for 2h, filter, and rinse the filter cake with absolute ethanol Wash, dry over anhydrous magnesium sulfate, obtain compound A;
[0027] (2) Dissolve 10g of compound A and 7.5g of sitosterol in 100mL of pyridine, the temperature is controlled at 35°C, then add 0.25g of 4-dimethylaminopyridine, carry out esterification reaction for 5h under stirring, filter, and extract the filtrate with hexane , the organic phase was washed successively with saturated brine, saturated sodium bicarbonate solution and water, dried with anhydrous calcium chloride and concentrated to remove the solvent to obtain a crude product, which was purified by silica gel column chromatography to obtain the target compound. The yield was 81.59%.
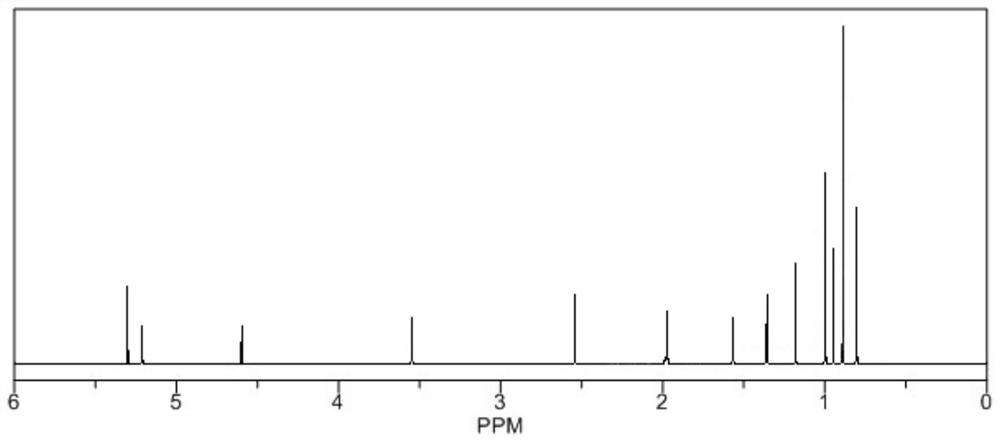
[0028] H NMR spectrum detection:
[0029] Put the sample into the sample tube, and inject 0.5mL CDCL3 (deuterated chlorofo...
Embodiment 2
[0030] Embodiment 2 The compound of the present invention is to the impact of absolute ethanol type ulcer in rats
[0031] Tested animals: SPF grade SD rats, body weight 180±20g, half male and half female. Before the test, the male and female mice were fed separately for 7 days and then the test was started. The room temperature was (25±2)°C, and the humidity was 67±13%.
[0032] Test method: healthy SD rats are randomly divided into normal control group (control group 1), model group (control group 2), cimetidine positive control group (control group 3), experimental group (embodiment 1 target compound ). The normal group and the model group were given equal volumes of distilled water, and the rest of the groups were fed for 7 days, once a day, fasted for 48 hours after the sixth administration, and 2 hours after the last administration, the rest except the normal control group Each group was fed with absolute ethanol, and then the rats were killed, their stomach tissues wer...
Embodiment 3
[0038] Embodiment 3 The effect of compound of the present invention on rat glacial acetic acid type gastric ulcer
[0039] SPF grade SD rats, fasted for 24 hours, anesthetized with 10% chloral hydrate injection, cut the abdominal wall and gently removed the rat’s stomach, and placed a glass tube with an inner diameter of 5mm vertically on the gastric serosa After 15 minutes, use a fine cotton swab to absorb the acetic acid in the glass tube, wipe it gently with a cotton swab of 0.9% sodium chloride solution for 2 times, and then cover the smeared surface of the acetic acid with the greater omentum, and lightly wipe the stomach Gently send it back to the abdominal cavity, and suture the peritoneal membrane and all layers of the abdominal wall. In the sham operation control group, only the stomach was pulled out for 15 minutes after laparotomy. The rats were randomly divided into a sham operation group (control group 1), a model group (control group 2), a cimetidine positive co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com