Anti-osteoporosis compound and derivative thereof, pharmaceutical composition, preparation method and application
An anti-osteoporosis and compound technology, applied in the field of anti-osteoporosis compounds and derivatives thereof, can solve the problem of uncertainty in taking estrogen, and achieve the effects of wide drug action targets, simple preparation method and excellent effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0130] Preparation of test cells:
[0131] C57BL / 6 mice were sacrificed by cervical dislocation, sterilized by immersion in 75% alcohol, the hind limb long bones (femur, tibia) were peeled off under sterile conditions, the attached soft tissues were removed, and the inner surface of the bone marrow cavity was repeatedly washed with complete medium to remove the cells in the bone marrow cavity completely. Washed out, the cell suspension was filtered with a cell sieve, and the cells were quantified and seeded in a 10 cm diameter culture plate under 5% CO. 2 The cells were cultured overnight under saturated humidity conditions, and the supernatant non-adherent cells were collected by centrifugation the next day, and replaced with fresh and complete medium (containing 30 ng / mL M-CSF) for two days to obtain bone marrow osteoclast precursor cells.
[0132] Inhibition rate (PR%)=[enzyme activity (negative control group)-enzyme activity (experimental group)] / [enzyme activity (negative...
Embodiment 1
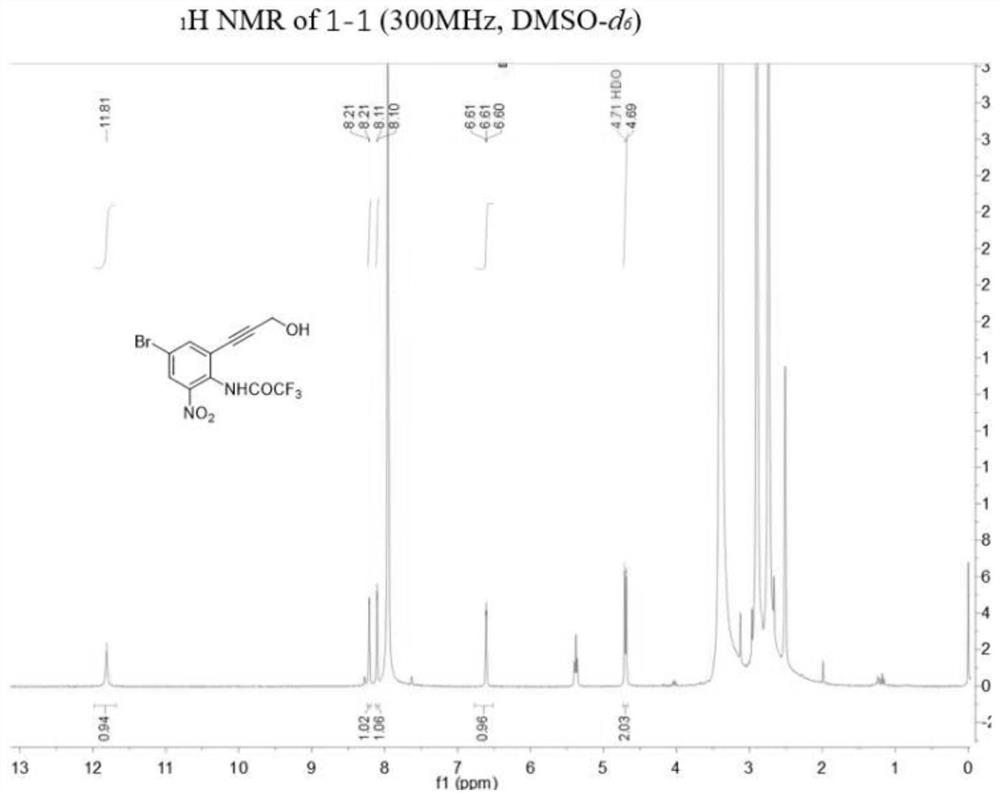
[0133] Example 1: N-(4-Bromo-2-(3-hydroxyprop-1-yn-1-yl)-6-nitrophenyl)-2,2,2-trifluoroacetamide (Compound 1- 1) Synthesis
[0134]
[0135] Among them: (a) Ag 2 SO 4 ,I 2 ,CH 3 OH, r.t., overnight; (b) (CF 3 CO) 2 O,anhydrous Et 3 N, anhydrous DCM, 0℃, 5min; r.t., 2h; (c) propargyl alcohol, PdCl 2 (PPh 3 ) 2 ,CuI,Et 3 N,DMF,N 2 ,r.t.,12h.
[0136] (1) Synthesis of 4-bromo-2-iodo-6-nitroaniline (compound 1a-1)
[0137] Add iodine (9.70 g, 38.23 mmol) to a 500 mL eggplant-shaped flask, add 250 mL of methanol to the flask to dissolve the iodine, then add silver sulfate (11.92 g, 38.23 mmol) and 4-bromo-2-nitroaniline in sequence (5.00 g, 25.49 mmol) and stirred at room temperature overnight. After the completion of the reaction was detected by TLC, the mixture was filtered and the reaction solvent was evaporated. After the residue was diluted with dichloromethane (100 mL), saturated sodium thiosulfate solution (100 mL) was added, then extracted with dichloromet...
Embodiment 2
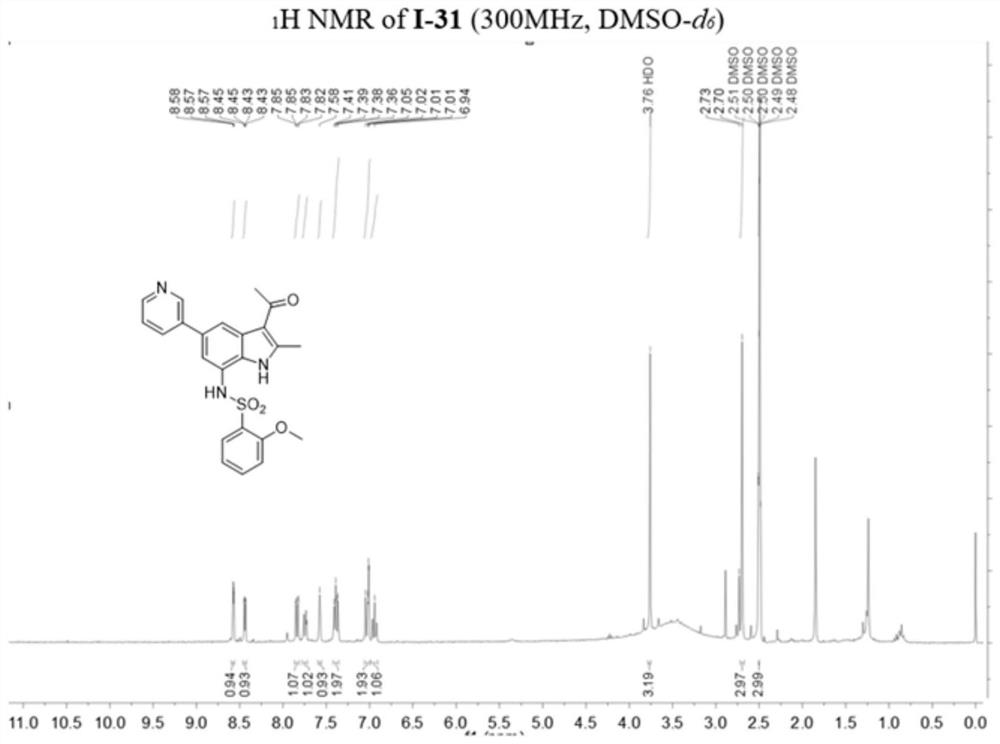
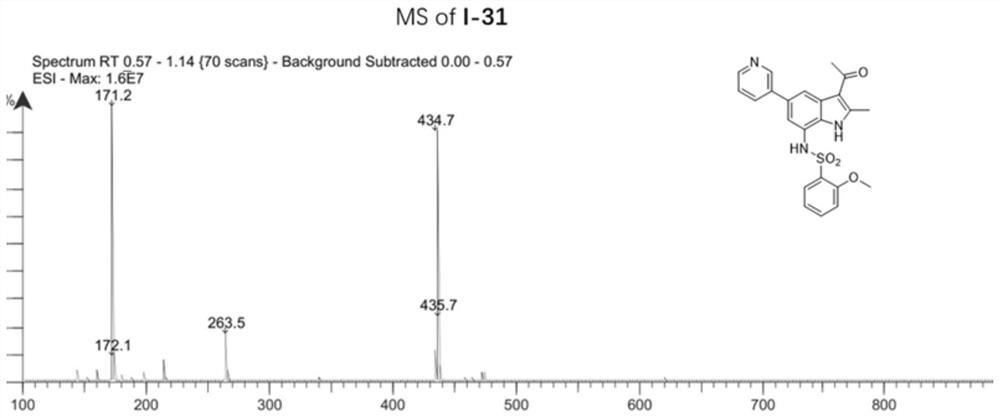
[0142] Example 2: 1-(7-(azetidin-3-ylamino)-2-methyl-5-(pyridin-3-yl)-1H-indol-3-yl)ethane-1 -Synthesis of ketone (compound 1-30)
[0143]
[0144] Of which: (a) 3-pyridylboronic acid, Pd (Ph 3 P) 4 ,K 2 CO 3 ,1,4-dioxane,H 2 O,100℃,12h;(b)ethyl chloroformate,anhydrous pyridine,0℃,5min;r.t.,2h;(c)Pd(PPh 3 ) 4 ,anhydrous Et 3 N,HCO 2 H,CH 3 CN,N 2 ,80℃,2h;(d)Pd / C,H 2 ,CH 3 OH / THF (v / v, 1 / 1), r.t., 5h; (e) EtOH, NaBH 4 ,rt,2h;(f)acetyl chloride,Et 2 AlCl, AlCl 3 ,N 2 , anhydrous DCM, 0℃, 5min; r.t., 5h; (g) CF 3 COOH,DCM,r.t.,1h.
[0145](1) 2,2,2-Trifluoro-N-(2-(3-hydroxyprop-1-yn-1-yl)-6-nitro-4-(pyridin-3-yl)phenyl)ethyl Synthesis of Amide (Compound 2-1)
[0146] Compound 1-1 (100 mg, 272.42 μmol), 3-pyridineboronic acid (49.8 mg, 408.63 μmol), tetrakis(triphenylphosphine)palladium (31.5 mg, 27.24 μmol) and potassium carbonate (113.0 mg, 817.26 μmol) were added Into a 25 mL three-necked round bottom flask, after replacing the air in the reaction system ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com