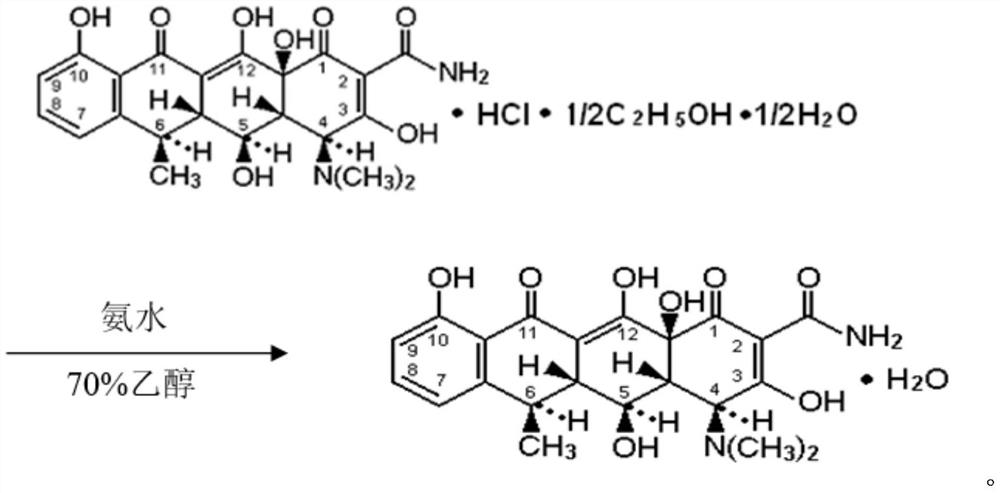
Preparation method of doxycycline monohydrate
A technology for doxycycline and doxycycline hydrochloride, which is applied in the field of compound preparation, can solve the problems of complex process, low yield, toxic sulfosalicylic acid, etc., and achieves stable material quality, high raw material yield and high technological simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The preparation method of doxycycline monohydrate is as follows:
[0035] (1) drop into 70% ethanol 1250Kg successively in the dissolution kettle of 2000L, drop into yellow doxycycline hydrochloride 500Kg under stirring, drop the temperature to 0 ℃ after throwing, start to drip ammoniacal liquor 200Kg slowly from the high position of ammoniacal liquor, keep the solution temperature over 10°C. When the material gradually changes from yellow to reddish brown, continuously measure the pH value with precision test paper (measured once every 3 minutes), control the pH ≤ 7.5, stir until completely dissolved, and continue stirring for 10 minutes.
[0036] (2) After confirming that the pH value remains unchanged, slowly add high-level pre-filtered CP hydrochloric acid under stirring, adjust the pH value to 6.00 at one time, heat the solution to 50°C while stirring, and keep it warm for half an hour to precipitate doxycycline Monohydrate crystals, turn on the cooling water to c...
Embodiment 2
[0038] The preparation method of doxycycline monohydrate is as follows:
[0039] (1) Put 1000Kg of 70% ethanol in a 2000L dissolving kettle successively, throw in 500Kg of yellow doxycycline hydrochloride under stirring, cool down to -5°C at the end of the throwing, start to slowly add 150Kg of ammonia water from the high position of ammonia water, and keep the solution temperature Not more than 10°C. When the material gradually changes from yellow to reddish brown, continuously measure the pH value with precision test paper (measured once every 4 minutes), control the pH ≤ 7.5, stir until completely dissolved, and continue stirring for 7 minutes.
[0040] (2) After confirming that the pH value remains unchanged, slowly add high-level pre-filtered CP hydrochloric acid under stirring, adjust the pH value to 6.50 at one time, heat the solution to 45°C while stirring, and keep it warm for half an hour to precipitate doxycycline Monohydrate crystals, turn on the cooling water to ...
Embodiment 3
[0042] (1) drop into 70% ethanol 1500Kg successively in the dissolving kettle of 2000L, drop into yellow doxycycline hydrochloride 500Kg under stirring, drop the temperature to 10 ℃ after throwing, start to drip ammoniacal liquor 250Kg slowly from the high position of ammoniacal liquor, keep the solution temperature over 15°C. When the material gradually changes from yellow to reddish brown, continuously measure the pH value with precision test paper (measured once every 5 minutes), control the pH ≤ 7.5, stir until it is completely dissolved, and continue stirring for 5 minutes.
[0043] (2) After confirming that the pH value remains unchanged, slowly add high-level pre-filtered CP hydrochloric acid under stirring, adjust the pH value to 5.50 at one time, heat the solution to 38°C while stirring, and keep it warm for half an hour to precipitate doxycycline Monohydrate crystals, turn on the cooling water to cool down and stir for 2 hours, then all the crystals will be precipita...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com