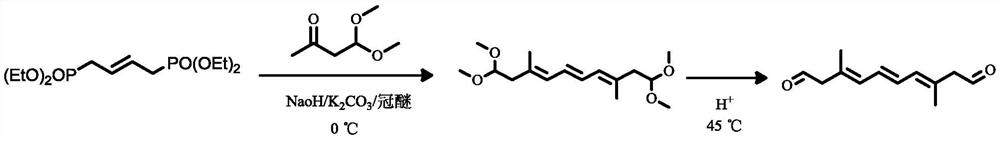
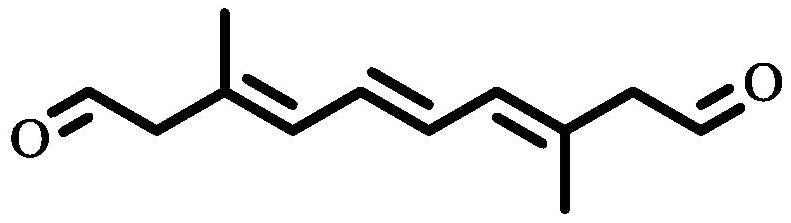
Method for synthesizing 3, 8-dimethyl-3, 5, 7-octatriene-1, 10-dialdehyde
A technology of octatriene and dimethyl, which is applied in the field of synthesizing 3,8-dimethyl-3,5,7-octatriene-1,10-dialdehyde, which can solve the problem of high cost and unreachable synthetic route Industrialization requirements and other issues, to achieve the effect of increased yield and mature process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A kind of synthetic method of 3,8-dimethyl-3,5,7-octatriene-1,10-dialdehyde, comprising the following steps:
[0035] Step 1: In a 200mL flask, add sodium hydroxide (1.92g, 48mmol), 6g of potassium carbonate and 50mL of a mixed solution of toluene and cyclohexane with a volume ratio of 1:1. The reaction flask was stirred at 0°C, and 2-butene-1,4-diphosphate tetraethyl ester (1.97g, 6mmol) and 4,4-dimethoxy-2- Butanone (3.17g, 24mmol) was added dropwise to the reaction flask, and 0.02g of 18-crown-6 was added. The reaction solution was reacted at 0° C. for 4 h. After the reaction was completed, 40 mL of water was added to wash, and the organic layer was taken by liquid separation, dried with anhydrous sodium sulfate, filtered, and the solvent was evaporated to dryness to obtain an intermediate product.
[0036] Step 2: Put the intermediate product obtained above in a 50 mL flask, add 4 mL of acetic acid, 2 mL of tetrahydrofuran, and 1 mL of water, stir and react at 45 ...
Embodiment 2
[0038] Step 1: In a 200mL flask, add sodium hydroxide (2.16g, 54mmol), 4g of potassium carbonate and 50mL of a mixed solution of toluene and cyclohexane with a volume ratio of 1:1. The reaction flask was stirred at 0°C, and 2-butene-1,4-diphosphate tetraethyl ester (1.97g, 6mmol) and 4,4-dimethoxy-2- Butanone (3.17g, 24mmol) was added dropwise to the reaction flask, and 0.03g of 18-crown-6 was added. The reaction solution was reacted at 0° C. for 4 h. After the reaction is completed, refer to the post-treatment method of Step 1 of Example 1 to obtain an intermediate product.
[0039] Step 2: Referring to Step 2 of Example 1, 0.43 g of the product 3,8-dimethyl-3,5,7-trienedialdehyde was obtained with a yield of 37.28%.
Embodiment 3
[0041] Step 1: Add sodium hydroxide (2.40 g, 60 mmol), potassium carbonate 2 g and 50 mL of a mixed solution of toluene and cyclohexane with a volume ratio of 1:1 in a 200 mL flask. The reaction flask was stirred at 0°C, and 2-butene-1,4-diphosphate tetraethyl ester (1.97g, 6mmol) and 4,4-dimethoxy-2- Butanone (3.17g, 24mmol) was added dropwise to the reaction flask, and 0.02g of 18-crown-6 was added. The reaction solution was reacted at 0° C. for 4 h. After the reaction is completed, refer to the post-treatment method of Step 1 of Example 1 to obtain an intermediate product.
[0042] Step 2: Referring to Step 2 of Example 1, 0.51 g of the product 3,8-dimethyl-3,5,7-trienedialdehyde was obtained with a yield of 42.80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com