3-O-aralkyl substituted 15-membered aza-lactone derivative and preparation method and application thereof
A technology of aralkyl derivatives, applied in the field of medicine, to achieve significant antibacterial activity and good antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
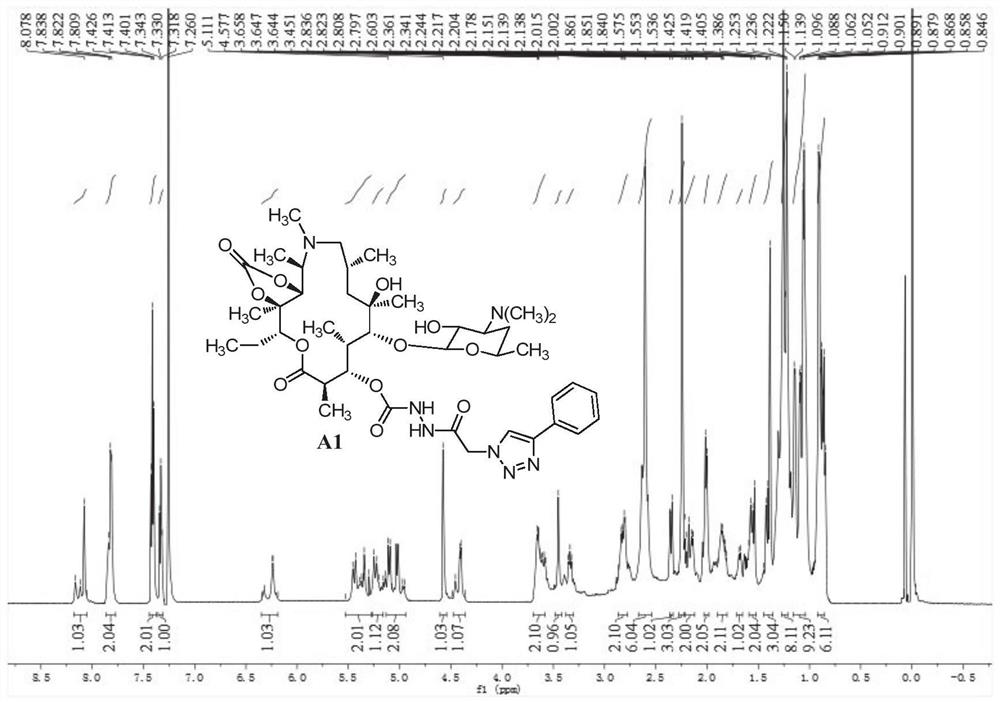
[0109] 3-O-[2-(4-Phenyl-1H-1,2,3-triazol-1-yl)acetylhydrazino-formyl]-3-O-descladinose Azithromycin-11,12 - Preparation of cyclocarbonate (A1)
[0110] 1. Preparation of 3-O-desclardinose azithromycin
[0111] Azithromycin (20 g, 26.72 mmol) was dissolved in methanol (200 mL), then the pH of the solution was adjusted to about 1 with hydrochloric acid (1 M), and stirred at room temperature for 24 hours. The reaction solution was concentrated under reduced pressure, the residue was dissolved in dichloromethane (200 mL), and the pH was adjusted to >7 with saturated sodium bicarbonate solution. Stand to separate the layers, wash the aqueous phase with dichloromethane (100 mL×2), and combine the organic phases. The organic phase was dried over anhydrous sodium sulfate, filtered with suction, and concentrated to obtain a crude product, which was then subjected to column chromatography (dichloromethane:methanol:ammonia=20:1:0.1~15:1:0.1~10:1:0.1) to obtain a white Solid 10.54g, yi...
Embodiment 2
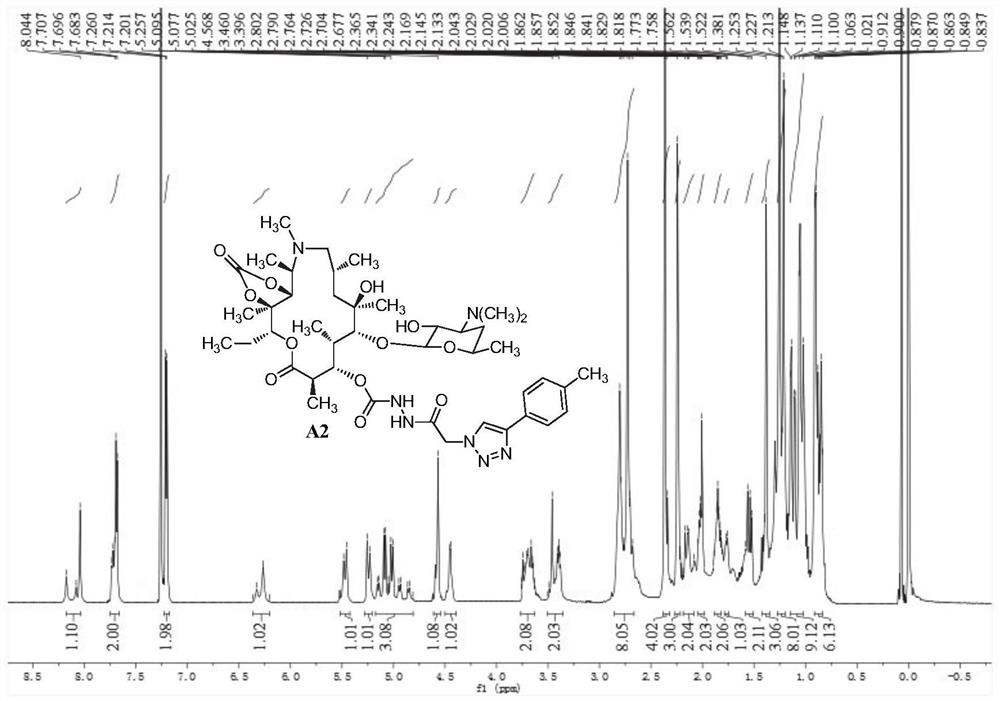
[0126] 3-O-[2-(4-p-Methylphenyl-1H-1,2,3-triazol-1-yl)acetylhydrazino-formyl]-3-O-declarinose Azithromycin- Preparation of 11,12-cyclocarbonate (A2)
[0127] A2 was prepared according to the preparation method of compound A1. White solid, melting point 143-145°C; 1 H NMR (600MHz, CDCl 3 ,δppm):8.18–8.04(m,1H),7.74–7.68(m,2H),7.21(d,J=7.8Hz,2H),6.36–6.21(m,1H),5.47(d,J=16.2 Hz, 1H), 5.24(d, J=16.2Hz, 1H), 5.16–4.81(m, 3H), 4.57(s, 1H), 4.45(d, J=6.8Hz, 1H), 3.76–3.63(m ,2H),3.50–3.38(m,2H),2.80–2.68(m,8H),2.37–2.34(m,4H),2.24(s,3H),2.19–2.08(m,2H),2.04–2.01 (m,2H),1.89–1.82(m,2H),1.77(d,J=8.9Hz,1H),1.58–1.52(m,2H),1.38(s,3H),1.25–1.21(m,8H ),1.15–1.02(m,9H),0.91–0.84(m,6H); MS(ESI)m / z calcd for C 43 h 68 N 7 o 12 [M+H] + :874.5,(C 43 h 69 N 7 o 12 ) 0.5 [M+2H] 2+ / 2:437.8,found:874.7,438.0.
Embodiment 3
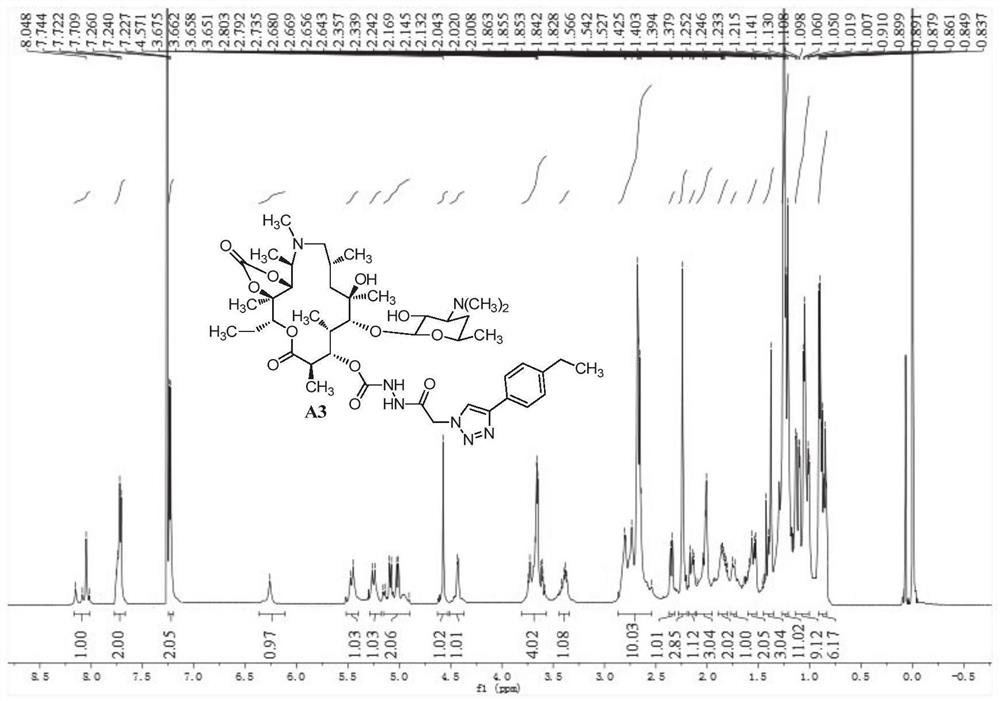
[0129] 3-O-[2-(4-p-Ethylphenyl-1H-1,2,3-triazol-1-yl)acetylhydrazino-formyl]-3-O-descladinose Azithromycin- Preparation of 11,12-cyclocarbonate (A3)
[0130] A3 was prepared according to the preparation method of compound A1. White solid, melting point 150-152°C; 1 H NMR (600MHz, CDCl 3 ,δppm):8.15–8.02(m,1H),7.76–7.71(m,2H),7.23(d,J=7.9Hz,2H),6.26(s,1H),5.46(d,J=16.3Hz, 1H), 5.25(d, J=15.7Hz, 1H), 5.16–4.90(m, 2H), 4.57(s, 1H), 4.43(d, J=6.6Hz, 1H), 3.74–3.58(m, 4H ),3.42–3.37(m,1H),2.86–2.54(m,10H),2.35(d,J=10.7Hz,1H),2.24(s,3H),2.17–2.13(m,1H),2.10– 2.01(m,3H),1.86–1.80(m,2H),1.74(d,J=14.5Hz,1H),1.61–1.53(m,2H),1.46–1.38(m,3H),1.25–1.22( m,11H), 1.08(ddd,J=54.6,22.7,6.7Hz,9H),0.91–0.84(m,6H); MS(ESI)m / z calcd for C 44 h 70 N 7 o 12 [M+H] + :888.5,(C 44 h 71 N 7 o 12 ) 0.5 [M+2H] 2+ / 2:444.8,found:888.7,445.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com