Method for leaching lithium from lepidolite
A lepidolite and leaching technology, which is applied in the field of lithium metallurgy, can solve the problems of unfavorable clean production environmental protection requirements, increase the cost of auxiliary materials, and environmental protection pressure, and achieve the effects of avoiding entrainment loss, improving purity, and reducing consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
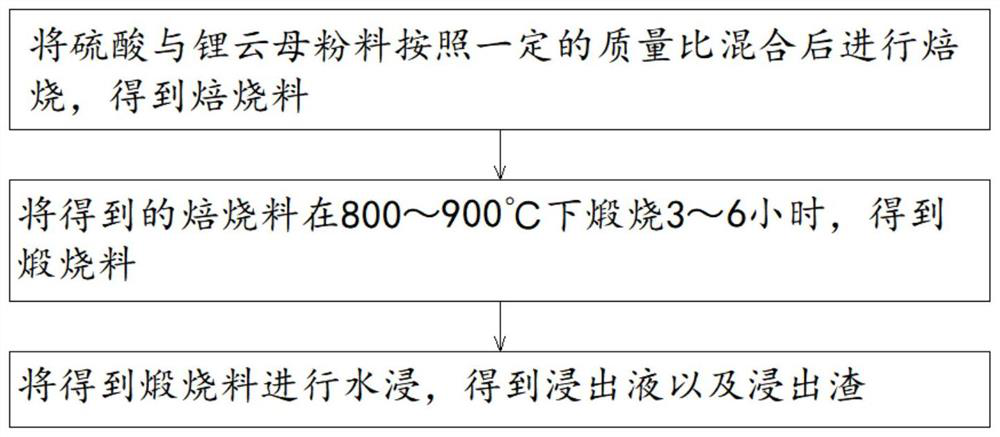
[0040] 1. Mix 98% sulfuric acid and lepidolite powder evenly at a mass ratio of 1.2:1; roast at 100°C for 1.5 hours to obtain a roasted material.
[0041] 2. After the calcined material is crushed appropriately (just crush the agglomerates), it is calcined at 880° C. for 4 hours to obtain the calcined material. The gas produced in the process is absorbed with sulfuric acid, and after the mass percentage of sulfuric acid reaches 98%, return to step 1 for sulfation roasting for batching.
[0042] 3. According to the solid-to-liquid ratio of 1:2, leaching the calcined material with water for 4 hours at room temperature, and filtering to obtain the leaching solution and leaching residue.
[0043] The leaching solution and leaching slag were taken for measurement, and the results of the leaching solution measurement are as follows:
[0044] analysis element Li Fe Ca Mg Al pH Leachate 8.03 <0.001
[0045] Lithium content in leaching residue: 0.14%. The le...
example 2
[0047] 1. Mix 98% sulfuric acid and lepidolite powder evenly at a mass ratio of 0.75:1; roast at 150°C for 2.5 hours to obtain a roasted material.
[0048] 2. After the calcined material is properly crushed (just crush the agglomerate), it is calcined at 900° C. for 6 hours to obtain the calcined material. The gas produced in the process is absorbed with sulfuric acid, and after the mass percentage of sulfuric acid reaches 98%, return to step 1 for sulfation roasting for batching.
[0049] 3. According to the solid-to-liquid ratio of 1:3, add water to leaching the calcined material at room temperature for 2.5 hours, and filter to obtain leaching liquid and leaching residue.
[0050] The leaching solution and leaching slag were taken for measurement, and the results of the leaching solution measurement are as follows:
[0051] analysis element Li Fe Ca Mg Al pH Leachate 6.03 <0.001
[0052] Lithium content in leaching residue: 0.16%. The lithium le...
example 3
[0054] 1. Mix 98% sulfuric acid and lepidolite powder evenly at a mass ratio of 0.9:1; bake at 150°C for 2 hours.
[0055] 2. After the calcined material is crushed appropriately (just crush the agglomerate), calcined at 800° C. for 5 hours to obtain the calcined material. Gases generated during the process are absorbed with sulfuric acid. After the mass percent of sulfuric acid reaches 98%, return to the first step for sulfation roasting batching.
[0056] 3. According to the solid-to-liquid ratio of 1:4, add water to leaching the calcined material at room temperature for 2 hours, and filter to obtain leaching liquid and leaching residue.
[0057] The leaching solution and leaching slag were taken for measurement, and the results of the leaching solution measurement are as follows:
[0058] analysis element Li Fe Ca Mg Al pH Leachate 4.25 <0.001
[0059] Lithium content in leaching residue: 0.12%. The lithium leaching rate was calculated to be 9...
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com