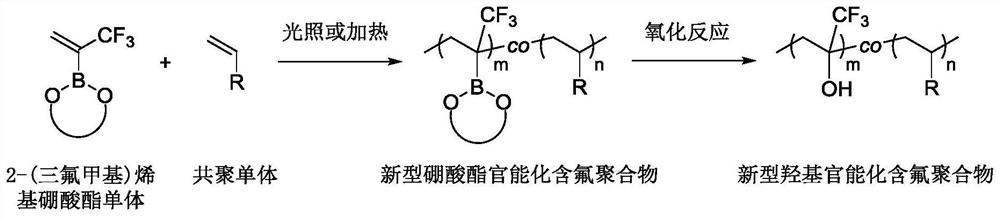
A kind of synthetic method of fluoropolymer rich in borate and hydroxyl
A technology rich in borate esters and fluoropolymers, applied in the field of polymer synthesis, can solve problems such as hindering the development of lithography technology and difficult to develop, and achieve a wide range of applicable monomers, fast polymerization reaction rate, and simple operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] In the embodiment, a kind of synthetic method of the fluoropolymer rich in borate and hydroxyl is provided, and the specific steps are as follows:
[0038] (1) Prepare the reaction solution: add the telogen, initiator, monomer and solvent into the reaction bottle respectively, stir and mix evenly, and remove the oxygen in the reaction system; the reaction solution is divided into four types:
[0039] Reaction solution A, containing the first monomer, the second monomer, thermal initiator, solvent;
[0040] The reaction solution B contains the first monomer, the second monomer, a thermal initiator, a solvent, and a telogen;
[0041] Reaction solution C, containing the first monomer, the second monomer, photoinitiator, solvent;
[0042] The reaction solution D contains the first monomer, the second monomer, a photoinitiator, a solvent, and a telogen;
[0043] In terms of molar ratio, monomer: initiator = 1000: (1~100); telomer is 0.0005~10 mol% of monomer
[0044] (2) ...
Embodiment 1
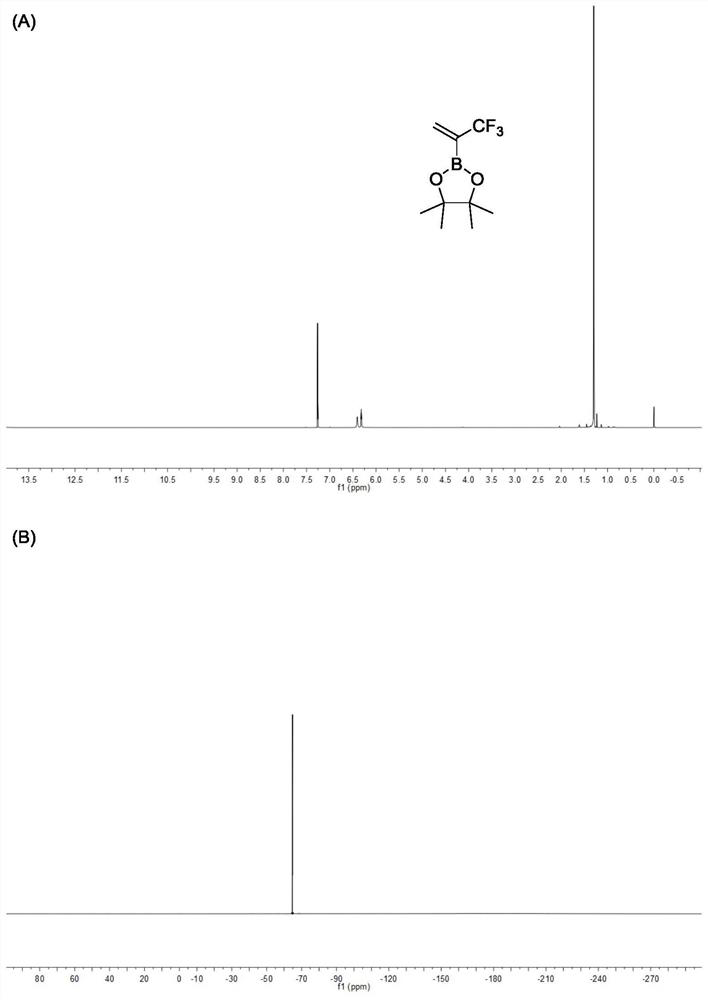
[0050] Example 1: A three-necked round-bottomed flask with a stirring bar was replaced with nitrogen three times and protected with nitrogen, and then 50 mmol of 2-bromo-3,3,3-trifluoropropene and 100 mL of tetrahydrofuran were added to the three-necked round-bottomed flask In the bottom flask, slowly add 47 mL of isopropylmagnesium chloride solution with a concentration of 1.3 mol / L containing lithium chloride dropwise at -78 °C, after the solution is added dropwise, stir for 5 hours, and then slowly add 60 mmol Methyl borate, turn off the refrigeration, continue to stir the reaction overnight, and slowly return to room temperature. After the reaction stopped, add 1mol / L hydrochloric acid solution for acidification, then add ether for extraction, combine the organic phases, then add 5g of anhydrous magnesium sulfate and 60 mmol of pinacol to the organic phase and continue stirring for 6 hours, then filter to remove the magnesium sulfate solid, a crude product was obtained, an...
Embodiment 2
[0052] Embodiment 2: Using reaction solution A, synthesize 2-(trifluoromethyl) vinylboronic acid pinacol ester and vinyl n-butyl ether copolymer by heating
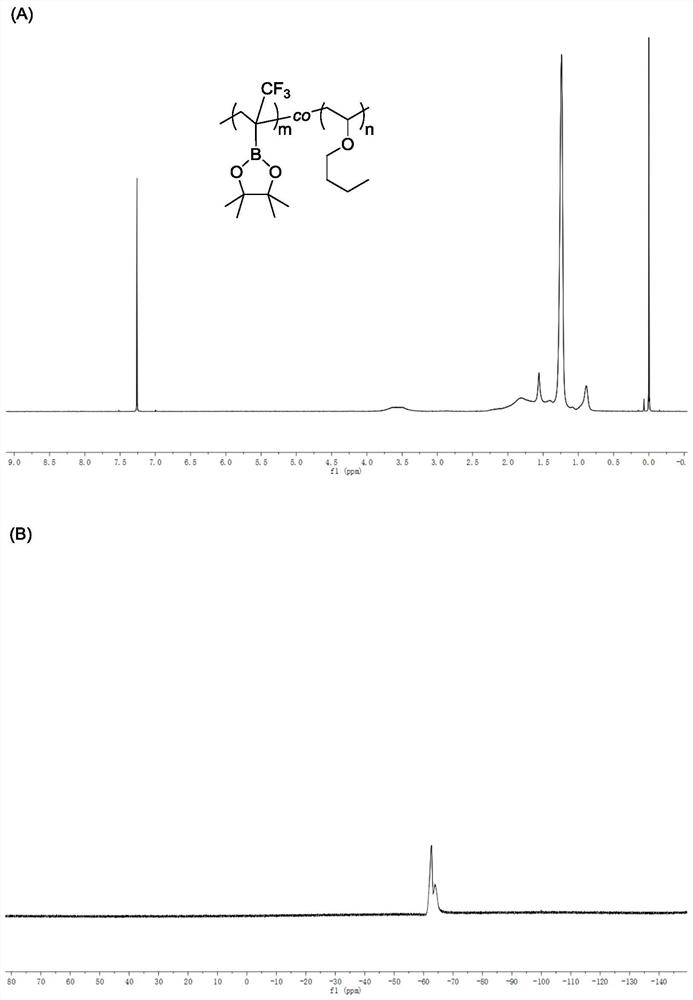
[0053] 50mmol, 50mmol and 0.5mmol of the above raw materials were added to the Add 50mL to the bottle with magnet N, N -Dimethylformamide, after stirring and dissolving evenly, the reaction mixture is deoxygenated, and then the reaction system is reacted at 65°C for 24 hours, and 2-(trifluoromethyl)vinylboronic acid is measured by hydrogen nuclear magnetic resonance The conversion rate of pinacol ester was 98%, and the conversion rate of vinyl n-butyl ether was 65%. The polymer sample was precipitated three times with a mixed solution of methanol and water with a volume ratio of 1:1, and dried in vacuum to constant weight to obtain a white solid. Polymer 1 HNMR spectrum, 19 The FNMR spectra are as follows image 3 (A), (B) shown. Polymer Molecular Weight by Gel Permeation Chromatography M n = 8.1 × 10 3 g / mol a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com