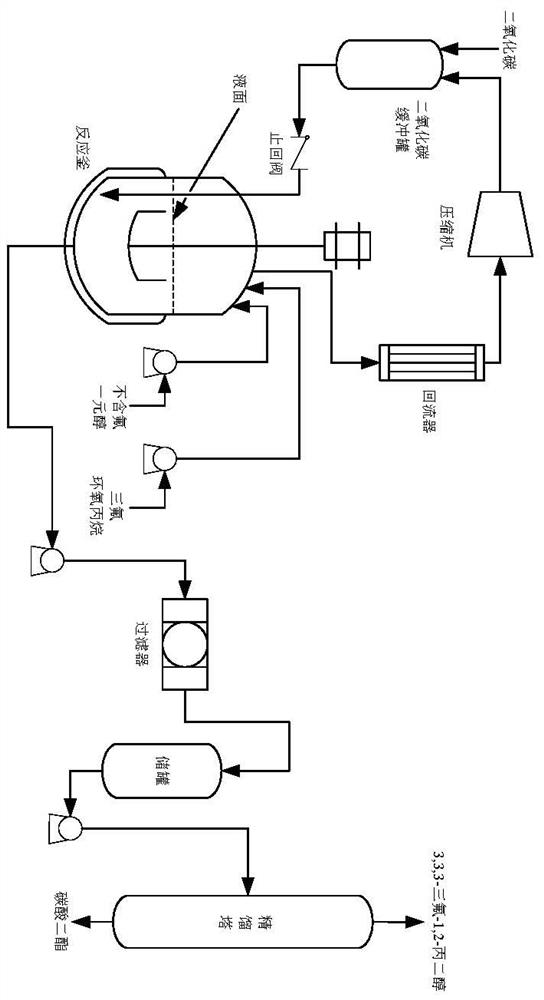
Preparation method of 3,3,3-trifluoro-1,2-propylene glycol
A technology of propylene glycol and trifluoropropylene oxide, applied in the field of fluorine chemical industry, can solve the problems of poor industrial application prospect, water or solvent consumption, poor safety, etc., and achieve the effects of short reaction time, few side reactions and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) Put 300g of trifluoropropylene oxide and 6g of solid catalyst (the amount is 2% of the mass of trifluoropropylene oxide) into a 1L high-pressure reactor, seal the reactor, and replace the air in the reactor with carbon dioxide;
[0037] (2) Stir the reaction kettle to raise the temperature to 45°C, and the stirring speed is 200-300r / min;
[0038] (3) Continuously bubbling carbon dioxide into the reactor until the pressure inside the reactor is 2.0~2.5MPa and maintained;
[0039] (4) After reacting for 6 hours, the unreacted carbon dioxide was discharged by releasing the pressure to obtain the intermediate product trifluoromethylethylene carbonate;
[0040] (5) Add 129g of methanol into the reactor, raise the temperature until the material boils, and reflux for 4 hours;
[0041] (6) After the reaction is completed, the products are separated by rectification to obtain dimethyl carbonate and 3,3,3-trifluoro-1,2-propanediol respectively. After weighing and testing, th...
Embodiment 2
[0043] (1) Put 300g of trifluoropropylene oxide and 6g of solid catalyst (the amount is 2% of the mass of trifluoropropylene oxide) into a 1L high-pressure reactor, seal the reactor, and replace the air in the reactor with carbon dioxide;
[0044] (2) Stir the reaction kettle to raise the temperature to 45°C, and the stirring speed is 200-300r / min;
[0045] (3) Continuously bubbling carbon dioxide into the reactor until the pressure inside the reactor is 2.0~2.5MPa and maintained;
[0046] (4) After reacting for 6 hours, the unreacted carbon dioxide was discharged by releasing the pressure to obtain the intermediate product trifluoromethylethylene carbonate;
[0047] (5) Add 185g of ethanol to the reaction kettle, heat up to boiling, and reflux for 4 hours;
[0048] (6) After the reaction is completed, the products are separated by rectification to obtain diethyl carbonate and 3,3,3-trifluoro-1,2-propanediol respectively. After weighing and testing, the selectivity is 99.4%, ...
Embodiment 3
[0050](1) Put 300g of trifluoropropylene oxide and 6g of solid catalyst (the amount is 2% of the mass of trifluoropropylene oxide) into a 1L high-pressure reactor, seal the reactor, and replace the air in the reactor with carbon dioxide;
[0051] (2) Stir the reaction kettle to raise the temperature to 45°C, and the stirring speed is 200-300r / min;
[0052] (3) Continuously bubbling carbon dioxide into the reactor until the pressure inside the reactor is 2.0~2.5MPa and maintained;
[0053] (4) After reacting for 6 hours, the unreacted carbon dioxide was discharged by releasing the pressure to obtain the intermediate product trifluoromethylethylene carbonate;
[0054] (5) Add 242g of propanol into the reaction kettle, raise the temperature until the material boils, and reflux for 5 hours;
[0055] (6) After the reaction is completed, the products are separated by rectification to obtain dipropyl carbonate and 3,3,3-trifluoro-1,2-propanediol respectively. After weighing and test...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com