Heavy metal precipitant, application, preparation method and acidic wastewater treatment method
A technology for heavy metal precipitation and polluted acid wastewater, which is applied in the fields of flocculation/precipitation water/sewage treatment, water/sludge/sewage treatment, multi-stage water treatment, etc. , copper, nickel ions and acid cannot be recycled and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The preparation method of zinc dimethyl dithiocarbamate is as follows: a complexing agent with a molar ratio of 2:1 and a zinc-containing inorganic salt solution are mixed and reacted, the reaction conditions are 25-30°C, and the reaction time is 2-4h; Wherein, the complexing agent is sodium dimethyl dithiocarbamate or potassium dimethyl dithiocarbamate, and the zinc-containing inorganic salt is zinc sulfate or zinc chloride; the dimethyl dithiocarbamate prepared by the above method Zinc yield is higher.
[0033] Preferably, the reaction condition is 30°C and the time is 2h.
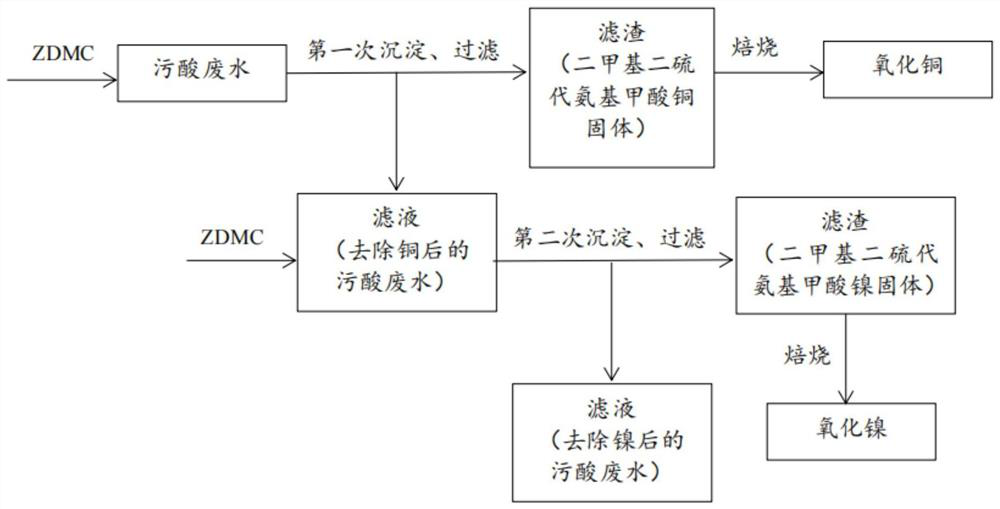
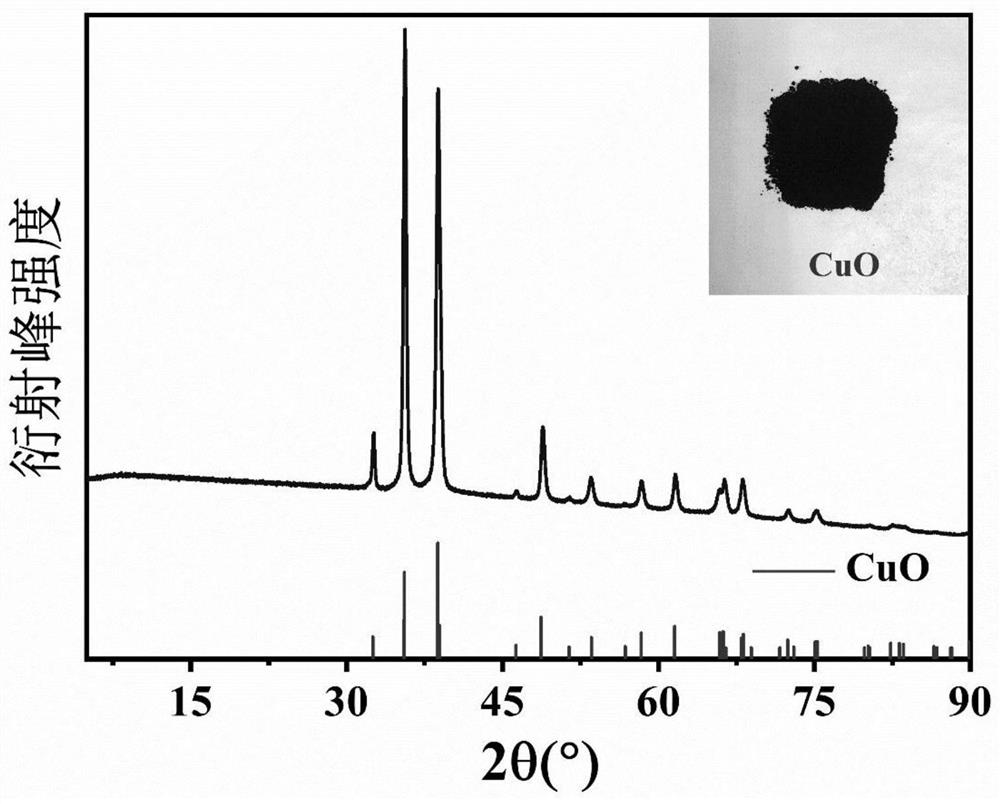
[0034] According to the method for treating polluted acid wastewater of the present invention, a heavy metal precipitant is added to the polluted acid wastewater to carry out the first precipitation, copper ions are reclaimed to obtain waste water for removing copper ions; Ions are recovered. like figure 1 shown, including the following steps:
[0035] Before the treatment, the concentrations ...
Embodiment 1
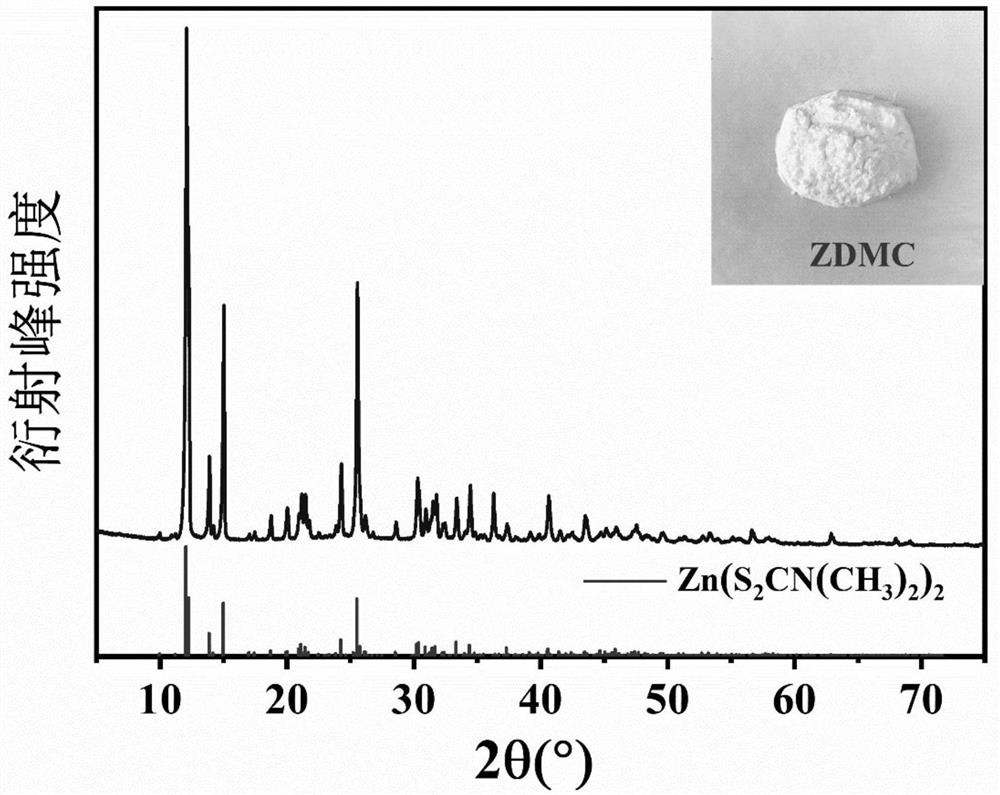
[0047] The heavy metal precipitant used in this example was prepared by the following method: 0.06mol sodium dimethyldithiocarbamate and 0.03mol zinc sulfate were used as raw materials, mixed uniformly, reacted at 30°C for 3h, filtered, and dried. Grind to obtain 9.05 g of zinc dimethyldithiocarbamate with a yield of 98.20%, which is sealed and stored for future use. figure 2 It is a diffractogram, wherein, the top spectrum is zinc dimethyl dithiocarbamate obtained in the present embodiment, and the corresponding spectrum below is the standard substance of zinc dimethyl dithiocarbamate. By comparison, it can be found that the zinc dimethyl dithiocarbamate spectrum obtained in this example is highly similar to the standard spectrum, indicating that the zinc dimethyldithiocarbamate obtained in this example has a higher purity.
[0048] Adopt processing method of the present invention to process 500mL polluted acid wastewater; Wherein, contain 1.02mol / L H in the polluted acid wa...
Embodiment 2
[0054] The heavy metal precipitant used in this example was prepared by the following method: 0.14 mol sodium dimethyldithiocarbamate and 0.07 mol zinc chloride were used as raw materials, mixed uniformly, reacted at 25 ° C for 2 h, filtered, and dried , and ground to obtain a total of 20.98 g of precipitant ZDMC, with a yield of 98.00%, which was sealed and stored for later use.
[0055] Adopt processing method of the present invention to process 5L dirty acid waste water; Wherein, contain 1.53mol / LH in the dirty acid waste water 2 SO 4 , 500mg / L Cu ions and 100mg / L Ni ions.
[0056] In the present embodiment, the addition amount of zinc dimethyl dithiocarbamate twice is 12.03g and 5.21g respectively.
[0057] The stirring conditions for the two times in this embodiment are both stirring at a frequency of 200 r / min at 30° C., and the stirring time is 180 min.
[0058] In this embodiment, after the first precipitation, the concentrations of Cu ions and Ni ions in the filtra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com