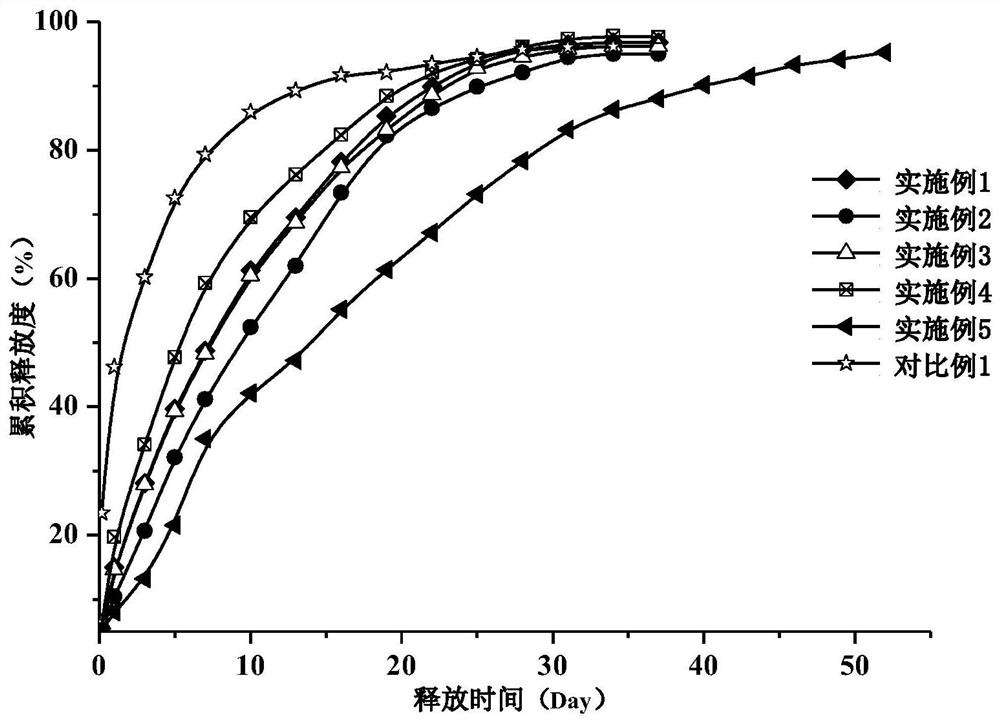
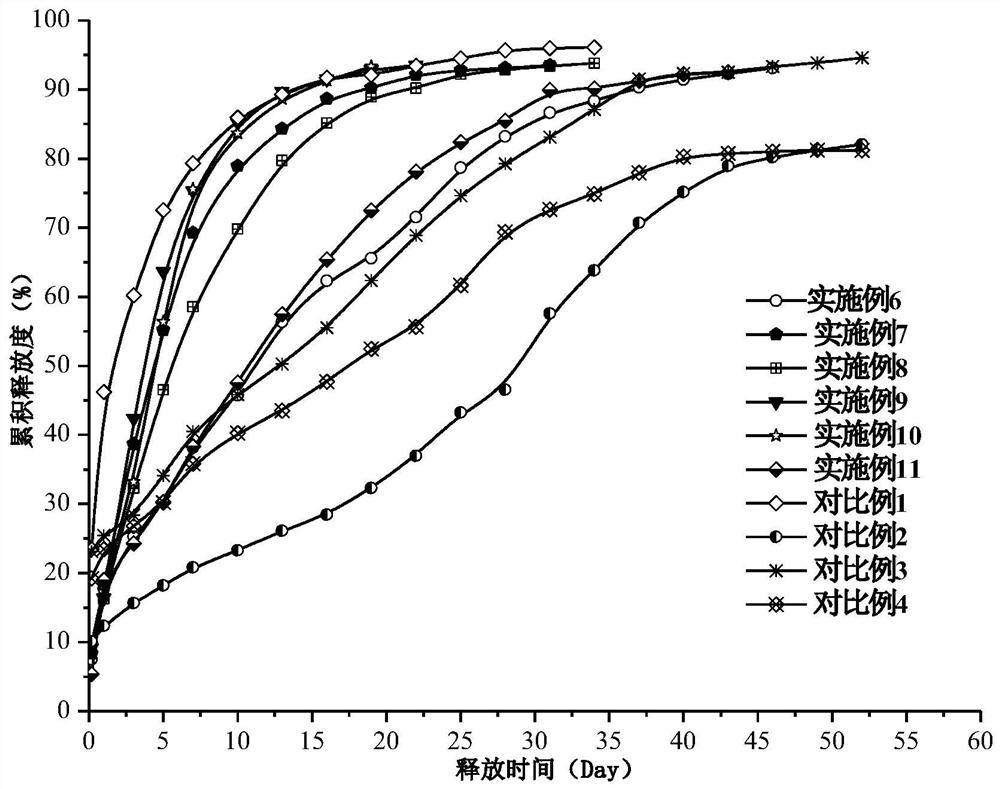
Dihydroxynaphthoic acid galanthamine sustained-release particle for injection and preparation method thereof
A technology of galanthamine pamoate and slow-release microparticles, which is applied in the field of galanthamine pamoate sustained-release microparticles for injection and its preparation, and can solve unfavorable process scale-up applications, low adsorption, and parameter deviation And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The preparation method of galantamine pamoate:
[0027] Dissolve 1.14 g of galantamine hydrobromide in 25 mL of dimethyl sulfoxide, add dropwise 100 mL of a dimethyl sulfoxide solution containing 1.41 g of pamoic acid, and after the drop is complete, stir and react at 25°C for 6 hours; Cool to 0-5°C and stand at this temperature for 24 hours, vacuum filter, and vacuum-dry at 50°C for 12 hours to obtain a light yellow powder which is galanthamine pamoate with a yield of 78.55% and a purity of 99.91% . All other raw materials and reagents used are commercially available.
[0028] In the embodiment, taking PLGA (75 / 25, 45000) as an example, it refers to a lactide-glycolide copolymer with a molar ratio of lactide to glycolide of 75:25 and a molecular weight of 45000; carboxyl-terminated, Both ester-terminated and hydroxyl-terminated lactide-glycolide copolymers can achieve the purpose of the present invention, and carboxyl-terminated lactide-glycolide copolymers are used ...
Embodiment 1
[0030] prescription:
[0031] PLGA (75 / 25, 45000) 35.892g
[0032] Galantamine Pamoate 8.975g
[0033] Arginine 0.135g
[0034] Preparation steps:
[0035] a. Mixing: Weigh the prescribed amount of PLGA, galanthamine pamoate and arginine and add them to a three-dimensional mixer for mixing. The working frequency is 25Hz and the time is 15min to prepare the mixture;
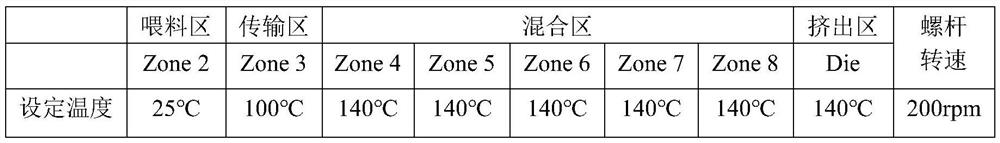
[0036] b. Melting and extruding: Add the mixture obtained in step a to the hopper feeding zone of the melting extruder. The mixture is heated in the conveying zone and then transported to the mixing zone. The air bubbles mixed in the product are extruded in the extrusion zone to cool the extrudate;
[0037] The parameters of the twin-screw extruder are as follows:
[0038]
[0039] c. Pulverization: put the extrudate obtained in step b in a ball mill, freeze it with liquid nitrogen for 10 minutes, grind it at -15°C to -5°C at a low temperature, work at a frequency of 30Hz, and pass it through a sieve of -9...
Embodiment 2
[0042] prescription:
[0043] PLGA (75 / 25, 45000) 35.82g
[0044] Galantamine Pamoate 8.955g
[0045] Lysine 0.225g
[0046] Preparation steps:
[0047] a. Mixing: Weigh the prescribed amount of PLGA, galanthamine pamoate and lysine and add them to a three-dimensional mixer for mixing. The working frequency is 25Hz and the time is 15min to prepare the mixture;
[0048] b. Melting and extruding: Add the mixture obtained in step a to the hopper feeding zone of the melting extruder. The mixture is heated in the conveying zone and then transported to the mixing zone. The air bubbles mixed in the product are extruded in the extrusion zone to cool the extrudate;
[0049] The parameters of the twin-screw extruder are as follows:
[0050]
[0051]
[0052] c. Pulverization: put the extrudate obtained in step b in a ball mill, freeze it with liquid nitrogen for 10 minutes, grind it at -15°C to -5°C at a low temperature, work at a frequency of 30Hz, and pass it through a sieve ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com