Ethylene oligomerization catalyst and method for continuously producing 1-hexene and 1-octene
An ethylene oligomerization and catalyst technology, which is applied in chemical instruments and methods, catalysts, and bulk chemical production, etc., can solve problems such as affecting the heat transfer of the reactor, clogging the pipeline, affecting the continuous operation time of the device, etc. The effect of reducing pipeline blockage and improving plant running time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
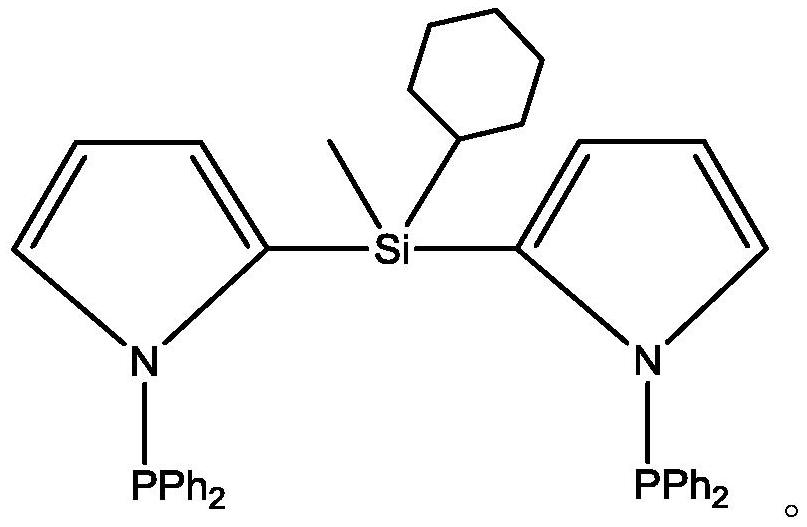
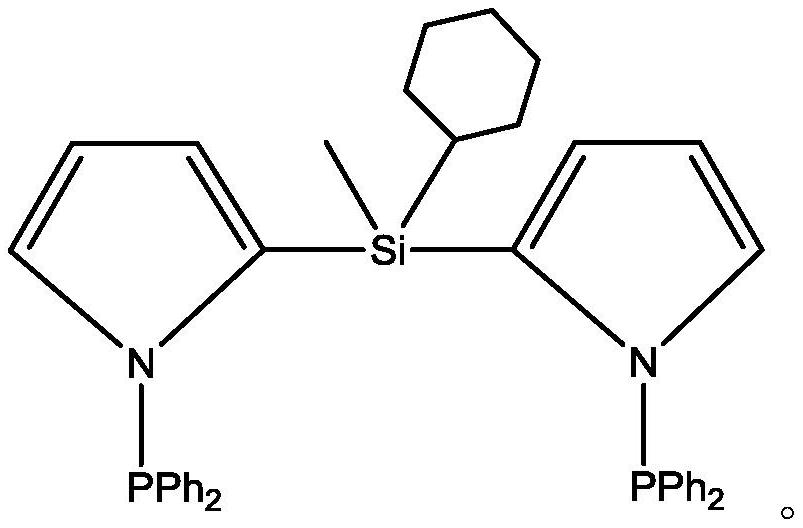
[0057] Ligand preparation: (1) Dissolve 4.5 mol of pyrrole in 400 ml of dichloromethane, and cool the mixed solution to -70°C; (2) Start stirring, and slowly add 4 mol of n-butyl lithium dropwise to the mixed solution successively After 10 minutes, add 2mol methylcyclohexyldichlorosilane and 0.22mol trifluoroacetic acid, react for 5 hours and place the mixed reaction solution at room temperature for 36 hours; (3) filter the insoluble matter in the mixed reaction solution, cool Slowly add 3.5 mol of diphenylphosphine chloride dropwise to the filtrate at -5°C, react for 3 hours and then place the reaction solution at room temperature for 6 hours; (4) Purify the reaction solution by column chromatography and rinse with tetrahydrofuran , the white solid powder obtained by evaporating the solvent is the PNSiNP ligand of the present invention, and the nuclear magnetic data of the above-mentioned ligand is as follows: 1H NMR (400MHz, CDCl ): 7.28~7.45 (m, 22H), 6.27~6.33 (m , 4H), 1....
Embodiment 2
[0067] Catalyst preparation: weigh 10mmol of CrCl 3 (THF) 3 , 20mmol of PNSiNP ligand and 18mmol of trityl tetrakis (pentafluorophenyl) borate, according to the method of Example 1, configure the methylcyclohexane of 0.5umolCr / mL into 20L with methylcyclohexane The solution was stored in a 30L catalyst storage tank under nitrogen protection.
[0068] Alkylaluminum compound dilution: 130g of triisobutylaluminum (20wt% Al) was diluted 200 times (mass ratio) with methylcyclohexane and stored in a 30L booster tank under high-purity nitrogen protection.
[0069] Continuous response:
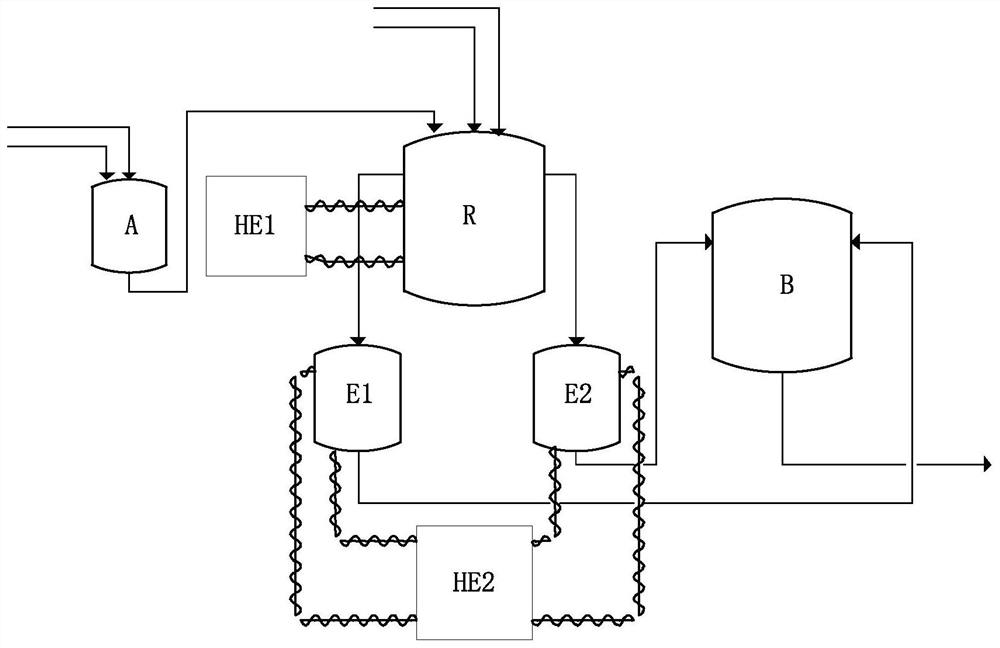
[0070] Such as figure 1 As shown, the continuous preparation of 1-hexene and 1-octene includes the following process:
[0071] Before the continuous reaction starts, the reaction kettle and the overflow tank reactor were heated to 130°C respectively, and the whole reaction system was purged with nitrogen for 7 hours. After the temperature dropped to room temperature, it was replaced with ethylene ...
Embodiment 3
[0076] Catalyst preparation: weigh 10mmol of CrCl 3 (THF) 3 , 18mmol of PNSiNP ligand and 40mmol of N,N-dimethylanilinium tetrakis (pentafluorophenyl) borate, according to the method of Example 1, configure 20L of 0.5umolCr / mL with methylcyclohexane Methylcyclohexane solution, stored in a 30L catalyst storage tank protected by nitrogen.
[0077] Alkylaluminum compound dilution: 110g of triisobutylaluminum (20wt% Al) was diluted 200 times (mass ratio) with methylcyclohexane and stored in a 30L booster tank under high-purity nitrogen protection.
[0078] Continuous response:
[0079] Such as figure 1 As shown, the continuous preparation of 1-hexene and 1-octene includes the following process:
[0080] Before the start of the continuous reaction, the reactor and overflow tank reactor were heated to 130°C respectively, and the entire reaction system was purged with nitrogen for 9 hours. After the temperature dropped to room temperature, it was replaced with ethylene for three ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com