Nucleic acid aptamer of protein, derivative of nucleic acid aptamer and application thereof
A nucleic acid aptamer and derivative technology, applied in the fields of biomedicine and analytical chemistry, to achieve the effects of good stability, high sensitivity, easy synthesis and transformation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
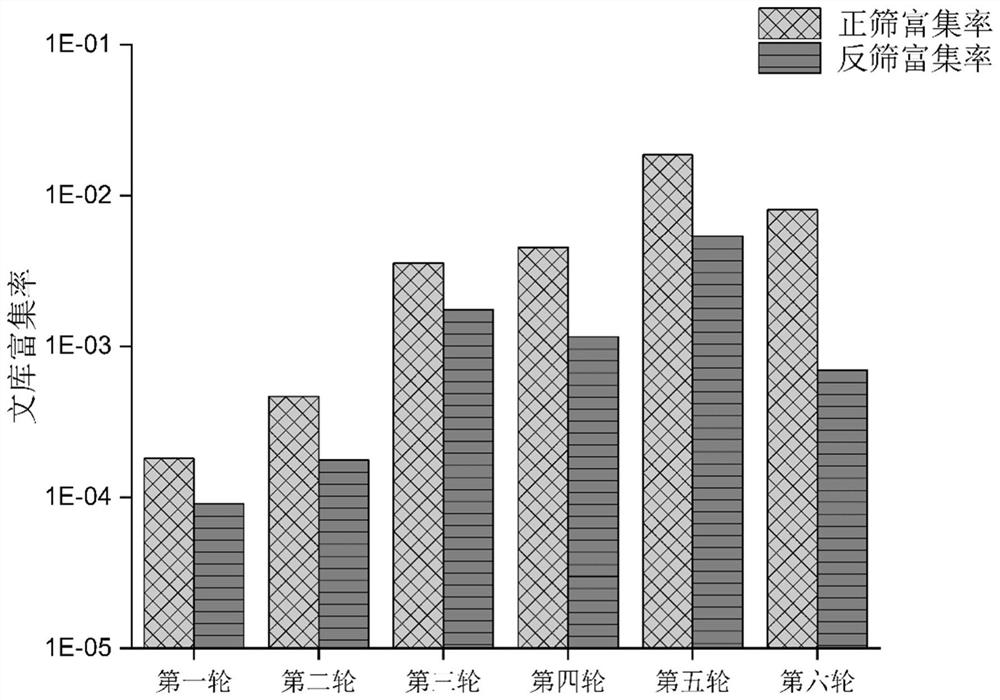
[0041] Example 1: Screening of NTRK1-specific nucleic acid aptamers
[0042] 1. Library reverse screening
[0043] 1.1 Take 25ug of His protein and incubate with 50ul of carboxyl magnetic beads activated by 0.4M EDC and 0.1M NHS at room temperature for 60min to couple to carboxyl magnetic beads; add 100ul of 1M ethanolamine and incubate at room temperature for 10min to quench unreacted Activate the carboxylic acid group, adsorb the magnetic beads on the magnetic frame, remove the supernatant, wash with an appropriate amount of DPBS 3 times, and set aside.
[0044] 1.2 Dissolve 1OD (about 1.4nmol) of the initial library in 280ul DPBS, heat at 95°C for 10min, cool in ice water for 5min, then place at room temperature for 5min, add His protein-coupled magnetic beads prepared in 1.1, and incubate at room temperature for 1h , the magnetic frame adsorbs the magnetic beads, and the collected supernatant is recorded as NTRK1-pool-1.
[0045] 1.3 Wash the magnetic beads 4 times with ...
Embodiment 2
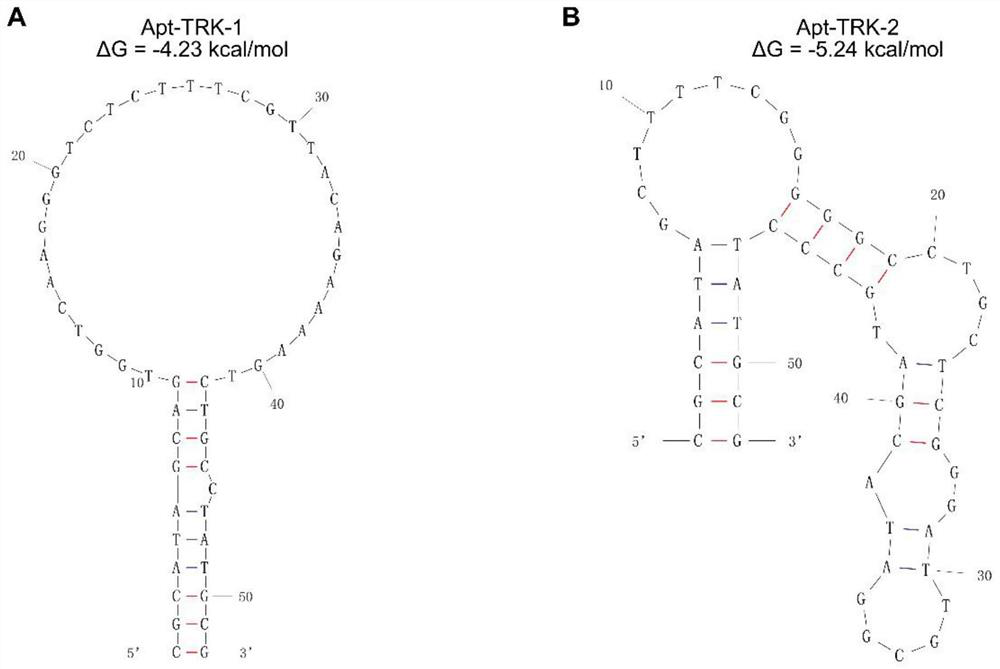
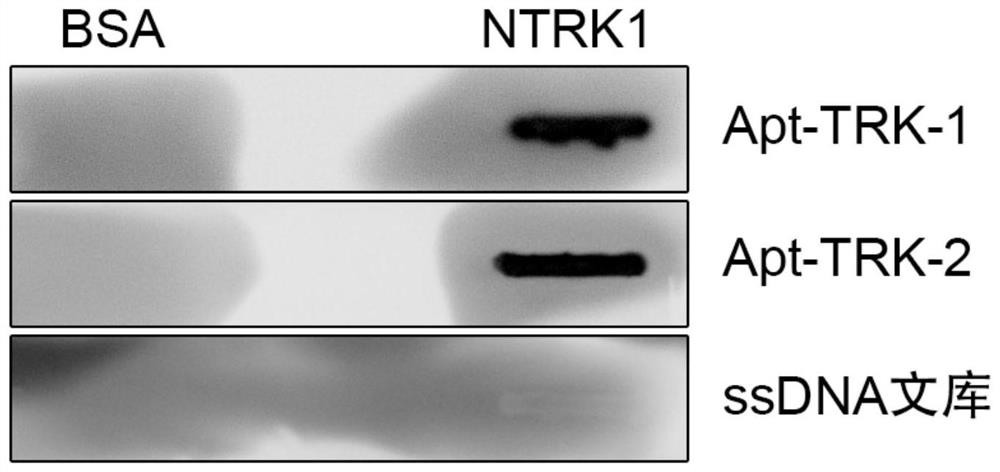
[0064] Example 2: Dot Blot detection of Apt-TRK-1 and Apt-TRK-2 binding to NTRK1 protein
[0065] The dot blot experiment was performed using a Smart Blotter vacuum transfer instrument (Wealtec, Smart Blotter SB-10). The filter paper and nitrocellulose membrane (NC membrane) were pre-cut, and the NC membrane was soaked with transfer buffer and placed on On the filter paper, put the NC membrane and filter paper on the support plate, and make sure there are no air bubbles on it, gently close the top cover, insert and close the flip stoppers on both sides at the same time, and then assemble the Smart Blotter system to the aspirator , spot 1ug NTRK1 protein in batches into the sample wells, use the same amount of BSA as the control protein, turn on the aspirator to pump air, wait for all the protein solution to pass through the membrane, turn off the aspirator after 5min, take out the membrane, and use 3 %BSA plus 0.1% Tween-20 was blocked at room temperature for 1 h, washed 3 tim...
Embodiment 3
[0066] Example 3: Determination of Apt-TRK-1 and Apt-TRK-2 and NTRK1 Protein Affinity
[0067] In order to detect the affinity between the screened nucleic acid aptamers and NTRK1 protein, we used the enzyme-linked nucleic acid aptamer adsorption method (ELASA) and the magnetic bead fluorescent quantitative PCR method to determine the level and dissociation constant K D .
[0068] 1. ELASA determination of the binding affinity of Apt-TRK-1 and Apt-TRK-2
[0069] In a 96-well ELISA plate, add 100ng NTRK1 protein to each well, coat with gentle rotation overnight at 4°C, discard the supernatant, wash 3 times with PBST, block with 3% BSA + 100ug / mL salmon sperm DNA for 2 hours, and aspirate Blocking solution, washed 3 times with PBST, added biotin-labeled Apt-NTRK1-1 and Apt-NTRK1-1 and Apt-NTRK1-1 and Apt- NTRK1-2, repeat 3 times in each well, add PBS as blank control at the same time, incubate at room temperature for 1 hour, aspirate the supernatant, wash 4 times with PBST, ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com