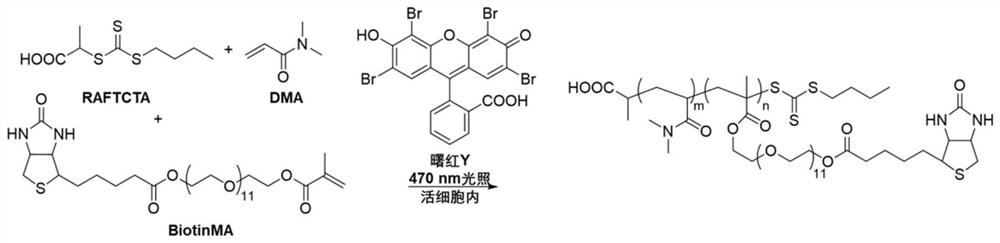
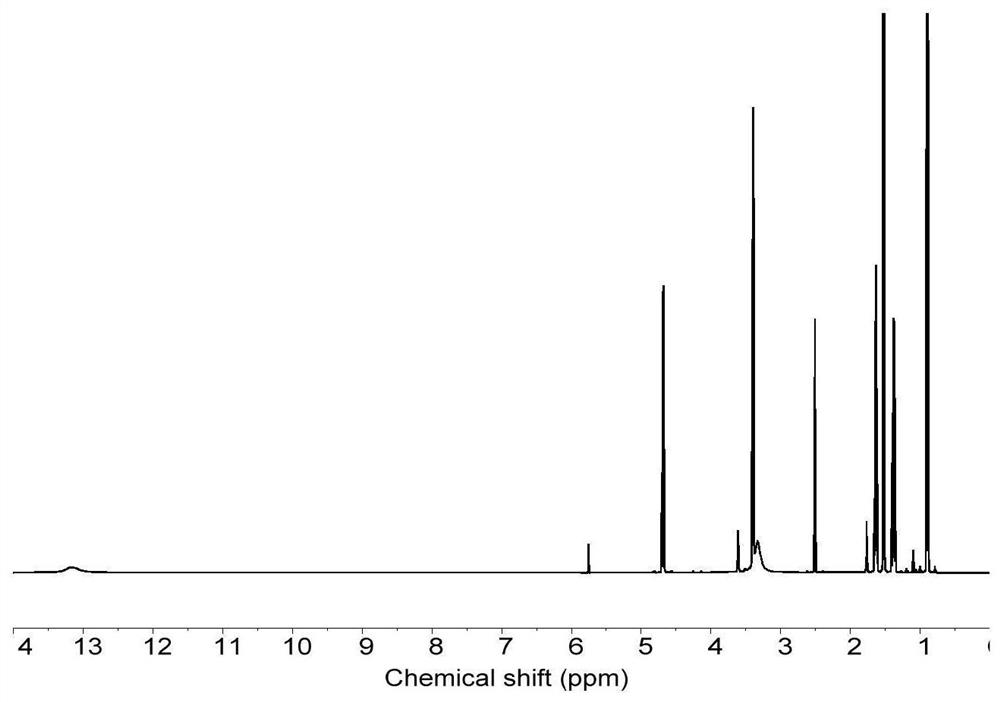
Composition and method for generating linear polymer through light-operated RAFT polymerization in living cells
A linear polymer and composition technology, applied in the field of cell biology, can solve the problems of small polymer molecular weight and uncontrollable reaction, and achieve the effect of low molecular weight distribution and low cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
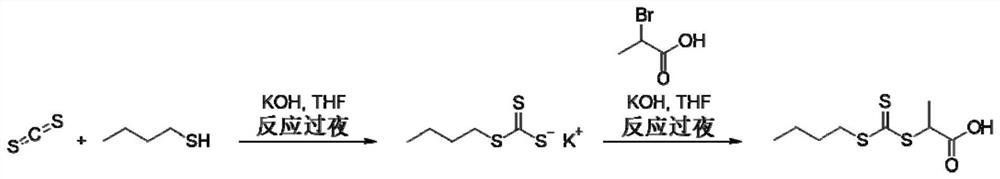
[0077] Embodiment 1: the synthetic steps of RAFT chain transfer agent 2-(butylthiocarbonylthiothiolthio)propionic acid
[0078] Such as figure 2 As shown, within 30min, add 50mL THF solution containing 16.0g n-butanol dropwise to 150mL THF suspension containing 9.0g KOH, stir at room temperature for 30min, then add dropwise 50mL solution containing 17.0g CS 2 After stirring at room temperature for 24 hours, the THF solution was concentrated to 50 mL under reduced pressure. Under stirring, 50 mL of THF solution containing 22.4 g of n-propylammonium bromide was added dropwise to the above concentrated solution. After stirring at room temperature for 24 hours, the solvent was removed under reduced pressure to obtain the target crude product. The product was further purified by silica gel column chromatography to obtain the target product as bright yellow crystals.
Embodiment 2
[0079] Example 2: Synthesis steps of biotin-modified acrylate monomer BiotinMA
[0080] Such as Figure 4 As shown, 1.0g biotin, 1.5g poly(ethylene glycol) methacrylate (Mn=500Da), 0.78g N-(3-dimethylaminopropyl)-N'-ethylcarbodiethylene Amine hydrochloride and 0.05g 4-(dimethylamino)pyridine were dissolved in 50mL DMF, stirred at room temperature for 24h, and the solvent was removed under reduced pressure. 3 , 1% hydrochloric acid and saturated brine for 3 times, dried with anhydrous sodium sulfate, and removed dichloromethane under reduced pressure to obtain the target crude product, which was further purified by silica gel column chromatography to obtain the target product as a white viscous liquid.
Embodiment 3
[0081] Example 3: CCK-8 test characterizes cell viability
[0082] In the CCK-8 test, HeLa cells were first treated with 1x10 4 The density was inoculated in a 96-well cell culture plate and incubated at 37°C and 4% carbon dioxide for 18 hours at a constant temperature to ensure that the cells adhered to the wall; 2 ), and continue to incubate at constant temperature for 24 hours; after 24 hours, suck out the original medium and wash the cells with PBS for 3 times, add 100 μL of diluted CCK-8 solution (ratio to medium: 1:10) to each well, incubate at constant temperature for 4 hours and use The microplate reader detects the ultraviolet absorption at 450nm in each well, and compares the absorbance of the treated cells with the untreated cells to obtain the cell activity value.
[0083] In the above method, through the CCK-8 test, it is proved that 10min of light and the composition will hardly cause toxicity to living cells within the working concentration range, as shown in t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com