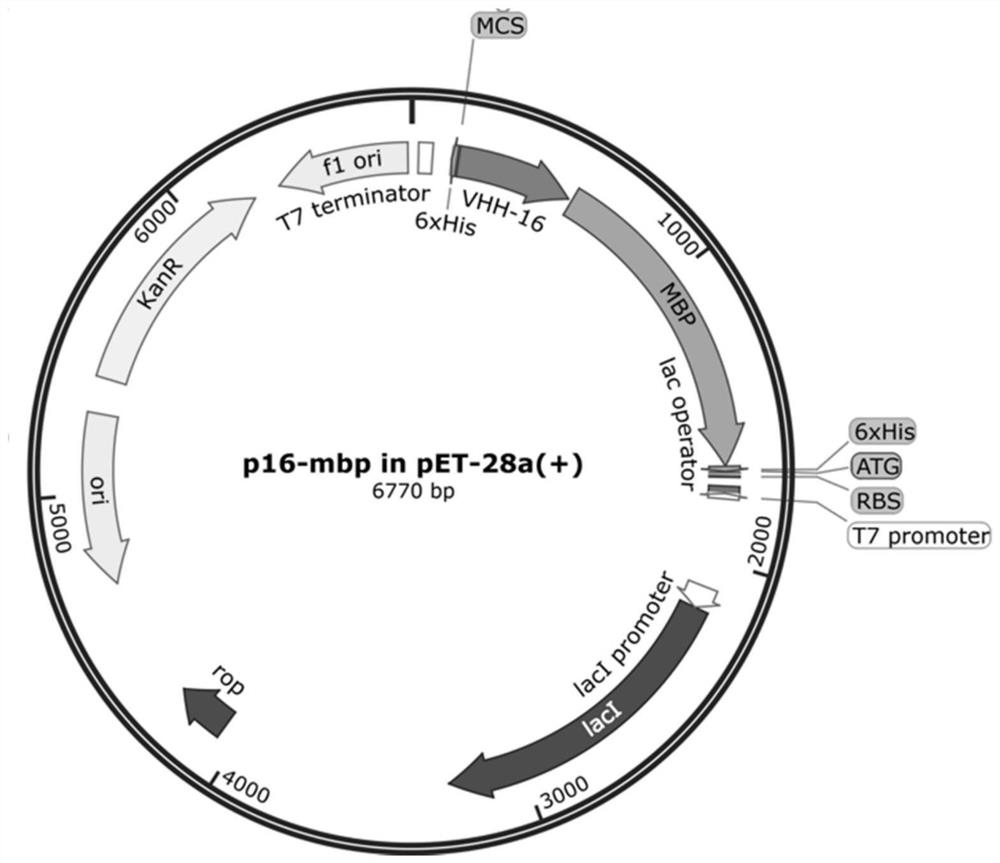
Novel anti-H5 subtype avian influenza virus nanoantibody and application thereof
An avian influenza virus and nanobody technology, used in antiviral agents, antiviral immunoglobulins, applications, etc., can solve the problems of long development cycle of monoclonal antibodies, difficult to obtain antibodies, and small size, and achieve good stability and biological stability. Chemical activity, improved accuracy and reproducibility, and high protein purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] The recombinant AIV inactivated vaccine (H5N1 subtype, RE-8 strain) avian influenza virus is immunized, once per three weeks, a total of immunization, extracting the peripheral lymphocyte of the immunization, separating the peripheral blood Lymphocyte (PBMC) was extracted for RNA, and the reverse transcription was obtained, and the VHH fragment was obtained by expansion of the second neocra PCR, and the VHH fragment was connected to the PCANTAB-5E phage vector, and the product was converted to XL- BLUE, constructs anti-protocarically targeted nano antibody libraries; assisted phage VCSM13 superfeiteria XL-BLUE bacteria, after overnight culture, collected upper and upper level can be obtained to obtain phage nanocarbon libraries.
Embodiment 2
[0061] The inactivated antigen H5 subtype avian flu (RE-8 strain) package was used on the enzyme label, 100 μL per hole (0.05 mol / l, pH 9.6). 10%, placed in 37 ° C for 2 h; discarded liquid, washed with 100 μl of PBS, add 300 μL of 3% BSA to be placed at room temperature to block 1h; discard the sealing liquid, add 100 μl of phage antibody fluid, incubate at room temperature for 1 h; Liquid and unbonded phage were washed 5 times with 0.05% PBST, 5 min / times, and 100 μl of glycine-hydrochloric acid pH = 2.2 was added, and the eluate was collected, and the amount of 2 mTris base was added, so that the liquid containing a phage In neutrality, the first round of enrichment screening phage; the phage is further amplified, and the next round of screening; the five-wheel "adsorption-eluting-amplification" screening process can eventually achieve strong specificity The phage clone (wherein the first round of screening adopts non-specific conditions, the latter round screening is impro...
Embodiment 3
[0063] Choosing a single colonite for ELISA method screening positive clone: a single colony is closed by a method package by the package to the enzyme label, with a blank control, and then add 100 μL / hole. HRP-M13, 37 ° C was incubated for 1 h, washed 5 times with 0.05% PBST, 5 min / times, add 100 μl / hole TMB substrate color color, and the protected room temperature was placed for 20 min, and the enzyme gauge was 620 nm OD value, with P / N value (i.e., the ratio of the positive hole OD reading / control hole OD reading) is greater than or equal to 3 is positive. The sequencing of positive clones can be obtained to obtain an anti-H5 subtype avian influenza nanobody sequence.
[0064] Its amino acid sequence is shown in SEQ ID NO.1: including the frame area (FR) and an antibody gene complementary determining region (CDR), the framework region is divided into four parts, sequentially defines FR1, FR2, FR3, FR4, sequentially recorded as SEQ ID No. 2, SEQ IDNO.3, SEQ ID No.4, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com